Figure 2.
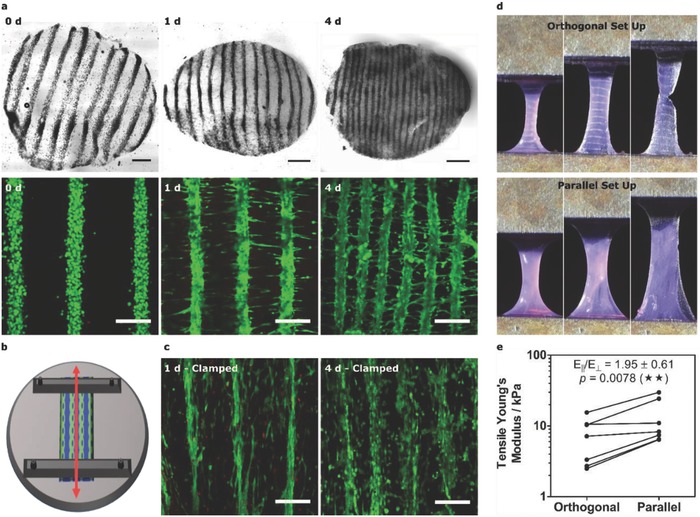
Engineering patterned muscle using collagen. a) Bright‐field and confocal fluorescence microscopy of acoustically patterned myoblasts in 3 mg mL−1 collagen. 4 mm diameter biopsy sections, isolated over 4 d, were stained with calcein (green, viable cells) and ethidium homodimer (red, nonviable cells). Bright‐field scale bars, 0.5 mm. Fluorescence scale bars, 200 µm. b) Schematic of mechanical clamping showing how imposed boundary conditions were used to generate static tensile load (red arrow) parallel with the patterned myoblast lines. c) Confocal fluorescence microscopy of the clamped constructs revealed cell‐level orientation and reduced interfiber contraction. Scale bars, 200 µm. d) Mechanical testing was performed with tensile strain applied either orthogonal or parallel with the cell lines. e) The tensile Young's modulus for the orthogonal and parallel configurations. Paired data from seven separate tissues (one‐tailed Wilcoxon matched pairs test), p ≤ 0.01 (**).
