Abstract
The impact of physical activity (PA) on lymphoma survival is not known. The association of PA and change in PA with overall (OS), lymphoma-specific (LSS) and event-free (EFS) survival was evaluated in a prospective cohort of newly diagnosed lymphoma patients (2002–2012).
We calculated Leisure Score Indexes (mLSI) from the self-reported usual adult PA (baseline) and at 3-years post-diagnosis (FU3), grouping patients by active versus insufficiently active by the American Cancer Society PA guidelines. Associations of PA with survival were assessed using hazard ratios (HRs) and 95% confidence intervals (CI) from Cox models stratified by lymphoma subtype, adjusted for age, sex, baseline BMI and comorbidity score with change scores further adjusted for baseline PA.
3,060 participants were evaluable at baseline and 1,371 at FU3. Active patients had superior survival from baseline [HR (CI): OS 0.82 (0.72–0.94); LSS 0.74 (0.61–0.90); EFS 0.92 (0.82–1.02)] and FU3 [HR (CI): OS 0.64 (0.46–0.88); LSS 0.32 (0.18–0.59); EFS 0.82 (0.61–1.10)] compared to insufficiently active. An increase in mLSI from baseline to FU3 (versus stable mLSI) was associated with superior OS (HR=0.70, CI 0.49–1.00) and LSS (HR=0.49, CI 0.26– 0.94).The continuous change in mLSI at FU3 was significantly associated with OS, LSS and EFS; maintained across subgroups and appeared linear.
Higher PA among lymphoma patients at diagnosis and 3-years is significantly associated with OS, LSS and EFS. Increasing PA after diagnosis is significantly associated with improved OS and LSS supporting an important role for PA in lymphoma survivorship and the need for intervention trials.
Keywords: Lymphoma survivors, Physical activity, Exercise
Introduction
It is estimated, as of January 1, 2016, there were 219,570 Hodgkin lymphoma (HL) and 686,370 non-Hodgkin lymphoma (NHL) survivors in the United States1. With an aging population and more effective therapies, the number of lymphoma survivors is expected to grow. There is increasing evidence that healthy behaviors benefit cancer survivors by improving fatigue, functioning, quality of life (QOL), risk of long-term complications and survival. Increasing physical activity (PA) may not just increase overall survival (OS) but also decrease risk of cancer progression or relapse in patients with cancers of the breast2,3, prostate4 and colon5–7. However, results from studies in solid tumor patients are difficult to extrapolate to lymphoma patients due to differences in disease biology, disease course, therapies utilized, and complications of treatment.
Healthy lifestyle recommendations from hematology-oncology providers can be a strong motivation for lymphoma survivors to adopt positive lifestyle changes8. It is known from population-based studies that pre-diagnosis smoking, alcohol use, obesity and low Vitamin D levels adversely affect lymphoma outcomes9–13. Pre-diagnosis physical activity (PA) in diffuse large B-cell lymphoma (DLBCL) has been associated with improved survival outcomes14. However, it is not known whether changes in PA after diagnosis can change lymphoma-specific outcomes in survivors.
We hypothesized that i) adults who are more physically active prior to and after lymphoma diagnosis have better lymphoma-related outcomes and ii) increasing the level of PA after lymphoma diagnosis can improve survival. Hence, we studied the association of self-reported usual adult PA, PA at 3 years after diagnosis and change in PA after diagnosis on OS, lymphoma-specific (LSS) and event-free survival (EFS) in a prospectively enrolled cohort of newly diagnosed patients with lymphoma.
Patients and Methods:
Study Cohort:
Full details of the Lymphoma SPORE Molecular Epidemiology Resource (MER), a prospective cohort study of newly diagnosed lymphoma patients aged 18 years and older, have been previously described15. This analysis includes participants from the Mayo Clinic in Rochester, Minnesota, who were also enrolled in a companion study of lymphoma etiology from 9/1/2002 through 12/31/201216. At enrollment, participants completed a baseline health and a selfadministered risk factor questionnaire (RFQ). The RFQ was part of the etiology study and included items on usual adult exercise, smoking, alcohol use and diet prior to the diagnosis of lymphoma. Pathology was reviewed by a hematopathologist and classified based on the WHO criteria17,18. Study personnel abstracted baseline clinical data and initial course of therapy. Responses from the MER cohort baseline questionnaire were used to calculate a baseline co-morbidity score, assigning 1 point each for the following self-reported conditions: other cancer diagnosis within 3 years of lymphoma diagnosis (except non-melanoma skin cancer), coronary artery disease, congestive heart failure, diabetes, hip fracture, hepatitis, autoimmune disease, and elevated creatinine.
All participants were contacted every 6 months for the first 3 years after diagnosis, and then annually thereafter to update health status. Disease recurrence or progression, new therapies, and new cancers were validated against medical records. For decedents, death certificates and medical records were reviewed by study physicians to assign cause of death. At 3 years after diagnosis, a survivorship questionnaire (FU3) that included items on PA, smoking, alcohol, and diet was sent to all participants. This was an observational study and patients received care at provider discretion with no specific PA intervention.
Participants were considered evaluable at baseline if they had completed baseline RFQ, including questions on usual adult PA. They were considered evaluable at FU3 if they had been evaluable at baseline and completed the FU3 questionnaire, including the Godin Leisure Time Exercise Questionnaire (Godin) 15. In order to minimize the effect of occult lymphoma recurrences on PA levels, patients who had events (disease recurrence, re-treatment or death) 6 months before or after FU3 were excluded from the FU3 and PA change analysis (CONSORT diagram Supplemental S1).
Assessment of physical activity
On the baseline RFQ, participants were asked “During most of your adult life, how often did you do strenuous/moderate/light exercise?” The questions listed examples of exercise by intensity and asked patients to exclude walking outside the home and PA associated with jobs.Participants answered on a 6-point frequency scale ranging from “rarely or never” to “5 or more days per week”. At FU3, participants completed the Godin, a validated tool for measuring PA in oncology patients19 (Supplemental S2). At FU3, participants also reported their perceived change in level of PA since lymphoma diagnosis by answering “How has your level of PA changed since your diagnosis of lymphoma or leukemia?” as no change, decreased or increased level of activity.
Statistical Analyses
We used PA data from the questionnaires to calculate a score corresponding to the Godin Leisure Score Index (LSI), which is a weighted summary measure of the frequency of self-reported weekly leisure-time exercise (times/week) calculated as (9 × strenuous activity) + (6 × moderate activity) + (3 × light activity) expressed in arbitrary units19. On the Godin, a moderate-to- strenuous LSI of ≥24 is used to classify cancer survivors into active and insufficiently active categories as per the American Cancer Society guidelines (≥ 150 minutes of moderate to vigorous physical activity per week)20. Although the RFQ did not have the specific Godin questions, we used the data on frequency, duration and intensity of PA to derive a modified LSI (mLSI). mLSI was modeled as a continuous score (per 10-point change) and by tertile. mLSI change was calculated as baseline mLSI subtracted from FU3 LSI. Survival was measured as time from diagnosis (for baseline PA) and time from FU3 (for FU3 PA and PA change from baseline to FU3 PA) until death due to any cause and due to lymphoma. For event analysis beyond FU3, participants who had an event prior to FU3 were excluded. We evaluated the association of PA with outcome using Kaplan-Meier curves as well as hazard ratios (HRs) and 95% confidence intervals (CI) from Cox models stratified by lymphoma subtype. All Cox models were adjusted for age, sex, baseline BMI and comorbidity score. The PA change models were also adjusted for baseline PA. Subset analyses stratified on age, body mass index (BMI), histological subtype, event free survival at 36 months (EFS36, defined as any lymphoma recurrence, complication due to lymphoma, or death due to lymphoma) and treatment status were also assessed. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results:
Usual PA prior to diagnosis and subsequent survival
From 2002 to 2012, 4,087 participants were enrolled in the MER at the Mayo Clinic, and of these, 3,129 participants completed a RFQ, of which 3,060 were evaluable for usual level of PA prior to diagnosis (baseline mLSI). The baseline characteristics of the evaluable patients are shown in Table 1. Compared to the participants who were evaluable for mLSI (N=3,060), the 1,027 participants enrolled but not evaluable for mLSI were more likely to be male (65% vs. 58%), have aggressive histology (44% vs. 37%), ECOG performance status ≥ 2 (9% vs. 5%), obesity (BMI ≥30, 35% vs. 29%) and missing co-morbidity data (31% vs. 20%) (Supplemental table S3). At baseline, the median mLSI was 28 (IQR 13–43); 1392 (46%) of patients could be classified as active (mLSI ≥ 24). Correlates of level of usual adult mLSI (active vs insufficiently active) are shown in Supplemental Table S4. Participants who were active had a lower median BMI and sufficient Vitamin D levels than those who were insufficiently active based on mLSI, else were similar on other factors.
Table 1. Baseline characteristics of patients evaluable for mLSI at baseline and at 3-year follow-up (FU3).
| Baseline cohort N= 3060 |
FU3 cohort N= 1371 |
|
|---|---|---|
| Median age at diagnosis (range), years | 62 (18–92) | 61(18–91) |
| Male | 1,770 (58%) | 771 (56%) |
| Race: Caucasian | 2,973 (98%) | 1339 (98%) |
| ECOG performance status: | ||
| Missing | 12 | 3 |
| <2 | 2,894 (95%) | 1319 (96%) |
| ≥2 | 154 (5%) | 49 (4%) |
| Co-morbidity score*: | ||
| Missing | 612 (20%) | 190 (14%) |
| 0 | 1962 (64%) | 951 (69%) |
| ≥1 | 486 (16%) | 230 (17%) |
| BMI: | ||
| Missing | 72 | 26 |
| <18.5 | 18 (1%) | 8 (1%) |
| 18.5–24.9 | 868 (29%) | 384 (28%) |
| 25.0–29.9 | 1202 (40%) | 533 (40%) |
| 30.0–34.9 | 579 (19%) | 277 (21%) |
| >/=35 | 318 (11%) | 143 (11%) |
| Stage: | ||
| Missing | 48 | 18 |
| Rai 0 | 422 (14%) | 211 (16%) |
| Rai I-II | 318 (11%) | 147 (11%) |
| Rai III-IV | 38 (1%) | 15 (1%) |
| Ann Arbor I-II | 854 (28%) | 387 (29%) |
| Ann Arbor III-IV | 1380 (46%) | 593 (44%) |
| B-symptoms: | ||
| Missing | 207 | 105 |
| Yes | 408 (14%) | 159 (12%) |
| No | 2,445 (86%) | 1107 (87%) |
| Histology: | ||
| Unclassified | 40 | 15 |
| Aggressiveε | 1131 (37%) | 462 (34%) |
| Indolentγ | 1889 (62%) | 894 (65%) |
MER co-morbidity score components (1 point each): other cancer diagnosis within 3 years of lymphoma diagnosis (except non-melanoma skin cancer), coronary artery disease, congestive heart failure, diabetes, hip fracture, hepatitis, autoimmune disease, and elevated creatinine. Missing co-morbidity score was used as a category for adjustment of Cox models.
Aggressive histologies: Diffuse large B-cell (DLBCL), Follicular (FL) grade 3, Burkitt, primary CNS, PTLD, high grade B-cell not otherwise specified, mediastinal B-cell, classical Hodgkin, peripheral T cell, angioimmunoblastic T cell, anaplastic large cell systemic, extra nodal NK/T cell nasal, enteropathy type T cell, Sezary syndrome, Precursor T/B lymphoblastic.
Indolent histologies: Chronic lymphocytic leukemia/Small lymphocytic lymphoma (CLL/SLL), Follicular grade 1 and 2, marginal zone, mantle cell, low grade lymphoma not otherwise specified, lymphoplasmacytic, mycosis fungoides, primary cutaneous B-cell, cutaneous T-cell, anaplastic large cell, large granular T-cell.
At a median follow-up of 8.9 years from diagnosis, there were 863 total deaths, 440 of which were attributable to lymphoma. Survival curves for mLSI by active vs insufficiently active are shown in Figure 1a. Compared to participants who were insufficiently active, those who were active had significantly superior OS (HR=0.82, 95% CI 0.72–0.94, p=0.004) and LSS (HR=0.74, 95% CI 0.61–0.90, p=0.003) with a trend towards better EFS (HR=0.92, 95% CI 0.82–1.02, p=0.114). Baseline mLSI modeled as a continuous score and by tertiles was significantly associated with OS, LSS and EFS (Supplemental figure S5a).
Figure 1:
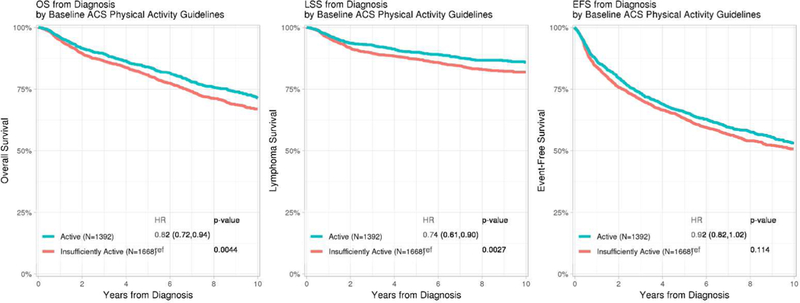
Kaplan-Meier plots of Overall, Lymphoma-specific and Event-free survival by a) baseline mLSI and b) FU3 LSI meeting versus not meeting the American Cancer Society physical activity guideline *HRs adjusted for age, sex, baseline BMI and comorbidity score
PA at FU3 and subsequent survival
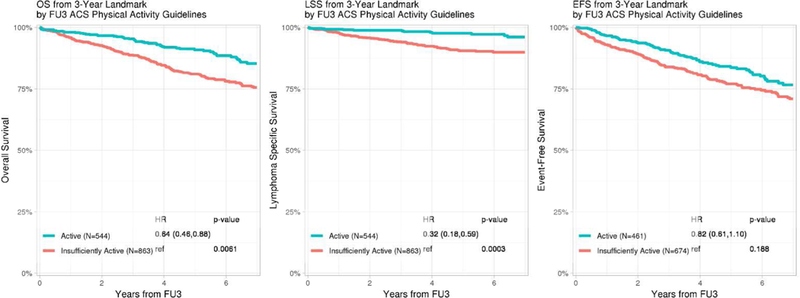
Of 3,060 participants with a baseline LSI, 368 died prior to FU3 and 93 who had an event within 6 months of FU3 were excluded. Baseline characteristics for those evaluable (N=1,371, Table 1) and not evaluable (N=1,228) for LSI at FU3 were comparable, except that co-morbidity information was more likely to be missing (26% vs. 14%) in those not evaluable (Supplemental Table S3). At FU3, the median LSI at FU3 was 23 (IQR 9–40); 544 (40%) of patients were classified as active (LSI≥ 24). Compared to the insufficiently active, FU3 survivors who were active were more likely to be younger, male, have aggressive lymphoma histology, lower BMI, fewer co-morbidities and higher baseline mLSI (Supplemental Table S4). For EFS analysis beyond FU3, participants who had an event prior to FU3 (N=267) were excluded and only participants who achieved EFS36 (N=1104) were included.
At a median follow-up of 2.3 years from FU3, there were 225 total deaths, 84 of which were attributable to lymphoma. Participants who were active had significantly better OS (HR=0.64, 95% CI 0.46–0.88, p=0.006) and LSS (HR=0.32, 95% CI 0.18–0.59, p<0.001) with a trend towards better EFS (HR=0.82, 95% CI 0.61–1.10, p=0.188) compared to those who were insufficiently active (Figure 1b). FU3 mLSI modeled as a continuous score and by tertiles was significantly associated with OS, LSS and EFS (Supplemental Figure 5b).
Change in PA from baseline to FU3 and subsequent survival
1,371 participants had both baseline mLSI and FU3 LSI available to assess change in PA and subsequent survival after FU3. The median change in mLSI from baseline to FU3 was −3 (IQR - 18 to +10). After accounting for baseline mLSI, the continuous mLSI change score (per 10-point change) was associated with significantly superior OS (HR=0.88, 95% CI 0.82–0.95, p=0.001), LSS (HR=0.74, 95% CI 0.65–0.84, p<0.001) and EFS (HR=0.92, 95% CI 0.85–0.98, p=0.016). The change in mLSI had an approximately linear association with OS, LSS and EFS, as confirmed by spline plots (Supplemental S6). Based on their change score, participants were divided into three groups: the highest (mLSI increase>5), middle (stable mLSI −12 to 5) and lowest (mLSI decrease < −12) tertiles. After accounting for baseline mLSI, category of change in mLSI was associated with OS and LSS (Figure 2a) but not EFS. Compared to patients with stable mLSI, patients with increased mLSI had marginally superior OS (HR=0.70, 95% CI 0.49–1.00, p=0.09), more strikingly superior LSS (HR=0.49, 95% CI 0.26–0.94, p=0.006) but not EFS (HR=0.79, 95% CI 0.57–1.10, p=0.282). There was no association of decreased mLSI with OS (HR=1.05, 95% CI 0.74–1.49), LSS (HR=1.56, 95% CI 0.90–2.71) or EFS (HR=1.04, 95% CI 0.73–1.48).
Figure 2:
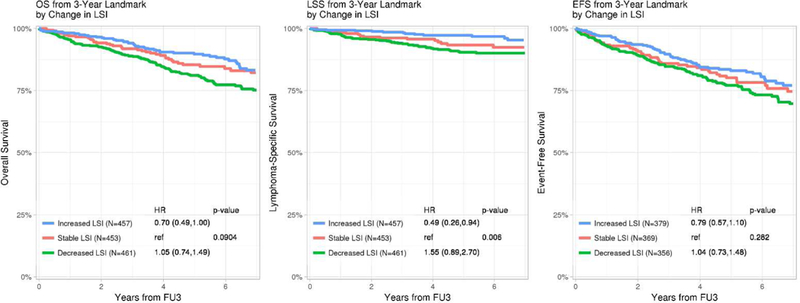
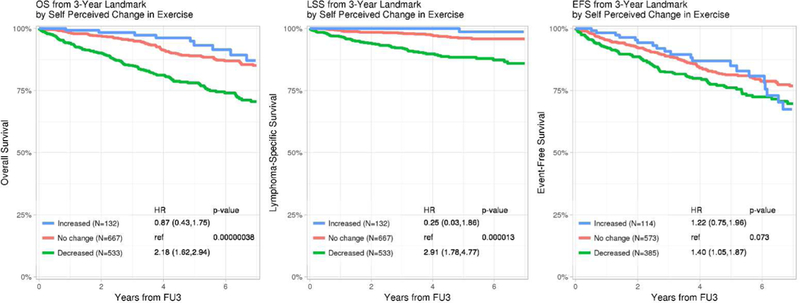
Kaplan-Meier plots of Overall, Lymphoma-specific and Event-free survival by a) change in mLSI from baseline to FU3 and b) self-perceived change in physical activity from baseline to FU3 *HRs adjusted for age, sex, baseline BMI, comorbidity score and baseline mLSI
Self-perceived change in PA from diagnosis to FU3 was also associated with OS, LSS and EFS (Figure 2b). Compared to no change, patients who perceived a reduction in their PA at FU3 had inferior OS (HR=1.93, 95% CI 1.50–2.47, p<0.001), LSS (HR=2.58, 95% CI 1.70–3.93, p<0.001) and EFS (HR=1.40, 95% CI 1.05–1.87, p=0.073), while a perceived increase in PA was not associated with OS (HR=0.86, 95%CI 0.49–1.50), LSS (HR=0.68, 95%CI 0.24–1.93) or EFS (HR=1.22, 95% CI 0.75–1.96), noting the latter estimate was based on a small number of events leading to wide confidence intervals.
Sub-group analysis:
In order to determine if the survival benefit from increasing PA was specific to any particular subgroup of patients, analyses by age (<60 vs. ≥60 years), sex (male vs. female), BMI (<30 vs. ≥30), co-morbidity score (0 vs. ≥1 vs. missing), disease histology (aggressive vs. indolent; individual subtypes DLBCL, CLL and FL), patients with and without events by FU3 (EFS36 achievers vs. non-achievers), and any treatment vs. no treatment by FU3 were performed. These results showed superior OS, LSS and EFS were consistently associated with higher mLSI at baseline and higher LSI at FU3 among all the subgroups analyzed (not shown). Figure 3 illustrates the subgroup analysis for change in mLSI from baseline to FU3 demonstrating a consistent survival benefit from increasing PA among all the lymphoma survivors.
Figure 3:
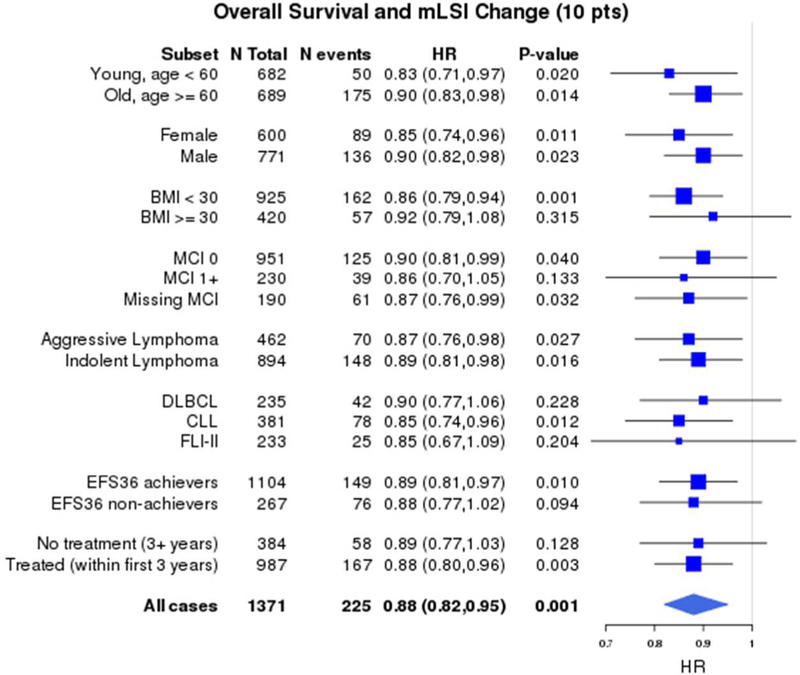
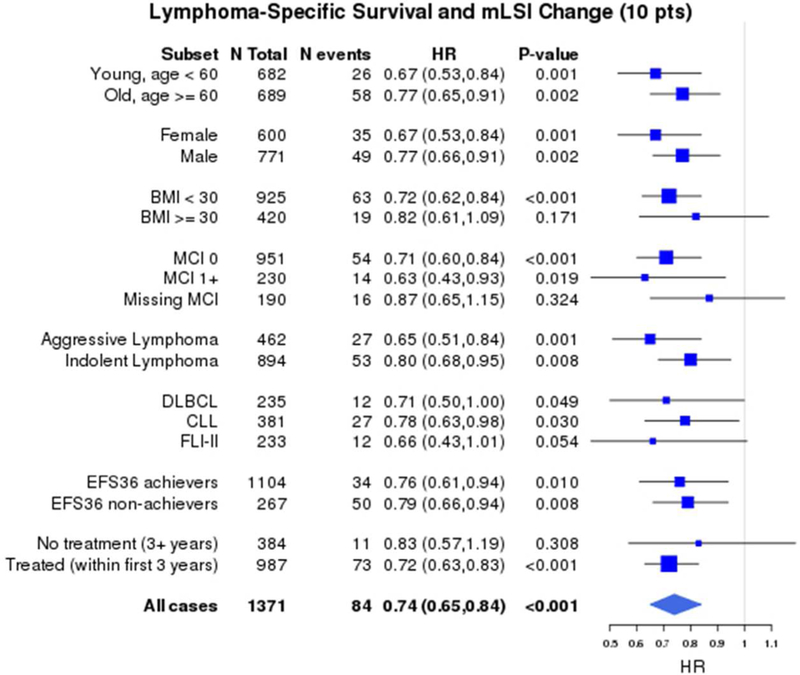
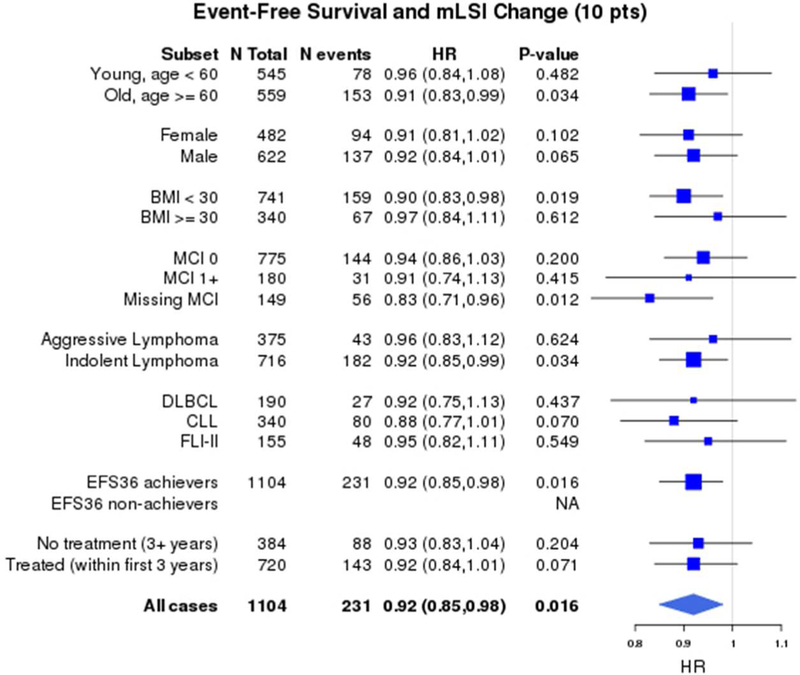
Forest plot of subgroup analyses of association of continuous change in mLSI (per 10- point change) with a) Overall survival b) Lymphoma-specific survival and c) Event-free survival *HRs adjusted for age, sex, baseline BMI, co-morbidity score and baseline mLSI
Discussion:
Our study shows that lymphoma patients with a higher level of usual PA during adult life prior to lymphoma diagnosis had significantly better OS and LSS after diagnosis compared to those who are less physically active. Higher level of PA in 3-year survivors was also associated with improved survival beyond the 3-year landmark. A change in the level of PA from baseline to FU3 had a stronger association with survival than baseline PA alone and this association was linear. Self-perceived decrease in PA from diagnosis to FU3 was associated with inferior survival. These associations held true irrespective of age, sex, co-morbidities, BMI, lymphoma histology at diagnosis, disease course and treatment.
The strengths of our study include prospective enrollment and follow-up of participants; detailed clinical data; measurement of PA at enrollment and at FU3, allowing assessment of change; ability to adjust for important potential confounding factors including level of comorbidity and BMI; and the length of follow-up. We do acknowledge several limitations. PA was self-reported, and thus is susceptible to measurement error, especially reporting of usual adult PA prior to diagnosis, which could be impacted by severity of lymphoma at enrollment. Further, we did not have the specific Godin questions for assessment of usual adult PA prior to diagnosis, as the Godin assesses PA in the past week; however, we were able to use data on frequency, duration and intensity of PA to develop a surrogate of the LSI. We also had many participants without available PA measurements at both enrollment and at FU3. While the differences between those with and without PA data at baseline are possibly affected by patients undergoing active work-up and management, we did not observe major differences in those with and without PA data at FU3. The calculated mLSI and self-perceived PA change results broadly paralleled each other even though they measured PA differently. While the change in PA provide compelling data to increase PA to improve outcomes in lymphoma patients, these observational data could be impacted by selection bias and unmeasured confounding factors, and the impact of a PA intervention needs to be addressed using a randomized clinical trial study design.
Survivorship guidelines as well as evidence for the role of health behaviors in lymphoma survivors are limited21. The only randomized clinical trial of exercise in lymphoma studied 122 patients in Canada22, with focus on physical functioning and QOL as study end points. Investigators randomized lymphoma patients on or off chemotherapy to usual care vs. 12 weeks of supervised aerobic exercise training and showed that exercise improves patient reported outcomes and objective physical functioning. A post-hoc analysis of this study did show a non-significant trend towards progression free survival benefit23. To our knowledge, our study is the first to measure the effect of change in PA after lymphoma diagnosis on subsequent OS and LSS. Recently, Boyle et al. reported that higher pre-diagnosis PA was associated with superior OS (HR=0.59, 95% CI 0.36–0.96) as well as superior LSS (HR=0.56, 95%CI 0.31–1.03) in 238 diffuse large B-cell lymphoma (DLBCL) patients, while PA was not associated with LSS in 175 follicular lymphoma (FL) patients14. Our results show that pre-diagnosis PA is significantly associated with improved OS and LSS, with similar results among individual subtypes (DLBCL, CLL, FL) and group (aggressive, indolent) in a much larger cohort. More importantly, this association holds true even 3 years after lymphoma diagnosis and PA seems to impact LSS to a greater degree than OS.
The mechanism by which PA affects OS in lymphoma patients may be explained in part by decreased cardiovascular events as is well established in the non-cancer population24. Jones et. al. studied 1,187 adult survivors of pediatric HL and found that vigorous intensity exercise was associated with a lower risk of cardiovascular events in a dose-dependent manner independent of cardiovascular risk profile and HL treatment25. The mechanism of disease-specific survival benefit from PA is likely multifactorial and not well understood 26,27 One possible explanation could be that patients who are more physically active are more likely to tolerate and complete lymphoma treatments23. Other explanations might include changes in metabolism, sex hormones, Vitamin D levels, angiogenesis, and immune function, although these have been mainly studied in breast or colorectal cancer patients to date12,13,28–31. Very few studies have examined the effect of PA on lymphoma biology, although there is a suggestion in animal models that tumor progression is retarded with PA via immunomodulatory mechanisms 32. Further studies are needed to elucidate mechanism of PA’s effects on prognosis for lymphoma survivors as we did see a trend towards improved event free survival with increased PA. A deficiency in Vitamin D has been associated with worse outcomes in certain lymphoma subtypes12,13,31. Vitamin D status, available only for 27% of the participants at baseline in our study, was associated with baseline PA levels and may be the biological link that warrants further investigation.
The American Cancer Society recommends at least 150 minutes per week of moderate intensity exercise or 75 minutes per week of vigorous intensity exercise for cancer survivors33,34. Two prior survey based observational studies, from US and Canada, studied PA and QOL in about 300–400 NHL patients. They found a positive association between meeting PA guidelines and QOL, but did not study survival outcomes35,36 . We found that all lymphoma patients (including CLL) meeting the exercise recommendations have an improvement in survival. However, it is important to emphasize the linear association between a positive change in PA and survival, indicating that any improvement in the level of PA has the potential to lower the risk of mortality. This is similar to results in solid tumors 37,38. It is not known if physical activity during treatment versus strictly post-treatment differs in outcomes, and interventional studies will need to be performed in this population.
Cancer survivors commonly ask what they can do to decrease the risk of recurrence or progression. Our study strongly suggests that providers should counsel patients on the important role for PA in lymphoma survivorship. Many efforts have been undertaken to improve upon the outcomes following standard chemotherapy in NHL, particularly in DLBCL, the most common subtype. With the exception of consolidative radiotherapy in bulky disease, most of these efforts have proven unsuccessful39. Herein, we found that PA is associated with improved overall and lymphoma-specific survival. These data provide a strong rationale for further investigating the role of PA in the care of lymphoma patients through intervention trials. Our study supports current national exercise guidelines for lymphoma survivors and suggests a benefit with even a modest increase in PA for those who cannot meet the guideline thresholds. Future studies are needed to elucidate the underlying biologic mechanisms as well as overcome barriers to effective delivery of exercise interventions to lymphoma patients.
Supplementary Material
Acknowledgments
Research Support: Lymphoma SPORE grant CA0972474
Footnotes
Previous presentation: Abstracts as an oral presentation at the American Society of Hematology Annual Meeting 2018, Atlanta, GA and poster presentation at Cancer Survivorship Symposium 2018, Orlando, FL
Disclaimers:None
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. [DOI] [PubMed] [Google Scholar]
- 2.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. [DOI] [PubMed] [Google Scholar]
- 3.Ligibel JAHLJ, Rugo HS, Burstein H, Toppmeyer DL, Anders CK, Ma C, Hudis CA, Winer EP, Barry WT Physical activity, weight and outcomes in patients receiving first-line chemotherapy for metastatic breast cancer: Results from CALGB 40502 (Alliance). Cancer Research. 2018;78(4). [Google Scholar]
- 4.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. [DOI] [PubMed] [Google Scholar]
- 6.Guercio BVA, Niedzwiecki D, Zhang S, Sato K, Fuchs C, Lenz HJ, Innocenti F, Fruth B, Van Blarigan E, O’Neil B, Shaw JE, Polite B, Hochster H, Atkins J, Goldberg R, Mayer R, Bertagnolli M, Blanke C, Meyerhardt J. Associations of physical activity with survival and progression in metastatic colorectal cancer: results from CALGB 80405 (Alliance). J Clin Oncol. 2017;35:suppl 4S; abstract 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid DLMF Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–1311. [DOI] [PubMed] [Google Scholar]
- 8.Jones LW, Courneya KS, Fairey AS, Mackey JR. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: a singleblind, randomized controlled trial. Ann Behav Med. 2004;28(2):105–113. [DOI] [PubMed] [Google Scholar]
- 9.Geyer SM, Morton LM, Habermann TM, et al. Smoking, alcohol use, obesity, and overall survival from non-Hodgkin lymphoma: a population-based study. Cancer. 2010;116(12):2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battaglioli T, Gorini G, Costantini AS, et al. Cigarette smoking and alcohol consumption as determinants of survival in non-Hodgkin’s lymphoma: a population-based study. Ann Oncol. 2006;17(8):1283–1289. [DOI] [PubMed] [Google Scholar]
- 11.Talamini R, Polesel J, Spina M, et al. The impact of tobacco smoking and alcohol drinking on survival of patients with non-Hodgkin lymphoma. Int J Cancer. 2008;122(7):1624–1629. [DOI] [PubMed] [Google Scholar]
- 12.Tracy SI, Maurer MJ, Witzig TE, et al. Vitamin D insufficiency is associated with an increased risk of early clinical failure in follicular lymphoma. Blood Cancer J. 2017;7(8):e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanafelt TD, Drake MT, Maurer MJ, et al. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood. 2011;117(5):1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle T, Connors JM, Gascoyne RD, et al. Physical activity, obesity and survival in diffuse large B- cell and follicular lymphoma cases. Br J Haematol. 2017. [DOI] [PubMed] [Google Scholar]
- 15.Cerhan JR LB, Habermann TM, Maurer MJ, Feldman AL, Syrbu SI, Thompson CA, Farooq U, Novak AJ, Slager SL, Allmer C, Lunde JL, Macon WR, Inwards DJ, Johnston PB, Micallef IN, Nowakowski GS, Ansell SM, Kay NE, Weiner GJ, Witzig TE. Cohort Profile: The Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) Cohort Study. International Journal of Epidemiology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerhan JR, Fredericksen ZS, Wang AH, et al. Design and validity of a clinic-based case-control study on the molecular epidemiology of lymphoma. Int J Mol Epidemiol Genet. 2011;2(2):95–113. [PMC free article] [PubMed] [Google Scholar]
- 17.Swerdlow SH, Campo E, Harris NL,Jaffe ES,Pileri SA, Stein H, Thiele J, Vardiman JW WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 18.Jaffe ES, Harris NL, Stein H, et al. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC, WHO Headquarters, Oxford University Press (USA); 2001. [Google Scholar]
- 19.Amireault S, Godin G, Lacombe J, Sabiston CM. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amireault S, Godin G, Lacombe J, Sabiston CM. Validation of the Godin-Shephard Leisure-Time Physical Activity Questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J Cancer Surviv. 2015;9(3):532–540. [DOI] [PubMed] [Google Scholar]
- 21.Ng AK. Current survivorship recommendations for patients with Hodgkin lymphoma: focus on late effects. Hematology Am Soc Hematol Educ Program. 2014;2014(1):488–494. [DOI] [PubMed] [Google Scholar]
- 22.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27(27):4605–4612. [DOI] [PubMed] [Google Scholar]
- 23.Courneya KS FC, Franco-Villalobos C, Crawford JJ, Chua N, Basi S, Norris MK, Reiman T. Effects of supervised exercise on progression-free survival in lymphoma patients: an exploratory follow-up of the HELP Trial. Cancer Causes Control. 2015;26:269–276. [DOI] [PubMed] [Google Scholar]
- 24.Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and metaanalysis. Eur J Cardiovasc Prev Rehabil. 2008;15(3):239–246. [DOI] [PubMed] [Google Scholar]
- 25.Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(32):3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betof ASDMW, Jones LW Effects and potential mechanisms of exercise trainng on cancer progression: A translational perspective. Brain Behav Immun. 2013;30(0):S75–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demark-Wahnefried WSKH, Alfano CM, BAil JR, Goodwin PJ, Thomson CA, Bradley DW, Courneya KS, Befort CA, Denlinger CS, Ligibel JA, Dietz WH, Stolley MR, Irwin ML, Bamman MM, Apovian CM, Pinto BM, Wolin KY, Ballard RM, Dannenberg AJ, Eakin EG, Longjohn MM, Raffa SD, Adams-Campbell LL, Buzaglo JS, Nass SJ, Massetti GM, Balogh EP, Kraft ES, Parekh AK, Sanghavi DM, Morris GS, Basen-Engquist K Weight management and physical activity throughout the cancer care continuum. Cancer. 2018;68(1):64–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betof ASLCD, Weitzel D, Landon C, Scarbrough PM, Devi GR, Palmer G, Jones LW, Dewhirst MW,. Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise. J Natl Canc Inst. 2015;107:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones LW, Antonelli J, Masko EM., Broadwater G, Lascola CD, Fels D, Dewhirst MW, Dyck JRB, Nagendran J, Flores CT, Betof AS, Nelson ER, Pollak M, Dash RC, Young ME, Freedland SJ Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol. 2012;113:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aleksandrova K, Jenab M, Leitzmann M, et al. Physical activity, mediating factors and risk of colon cancer: insights into adiposity and circulating biomarkers from the EPIC cohort. Int J Epidemiol. 2017;46(6):1823–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drake MT, Maurer MJ, Link BK, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(27):4191–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verma VK, Singh V, Singh MP, Singh SM. Treadmill exercise-dependent tumor growth retardation in T-cell lymphoma-bearing host displays gender dimorphism. Oncol Res. 2010;18(7):293–304. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz KHCKS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. [DOI] [PubMed] [Google Scholar]
- 34.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. [DOI] [PubMed] [Google Scholar]
- 35.Keith M Bellizzi JHR, Arora Neeraj K., Hamilton Ann S., Miller Melissa Farmer, Aziz Noreen M.. Physical Activity and Quality of Life in Adult Survivors of Non-Hodgkin’s Lymphoma. Journal of Clinical Oncology. 2009;27(6):960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VALLANCE JKCK, JONES LW, REIMAN T Differences in Quality of Life Between Non-Hodgkin’s Lymphoma Survivors Meeting and Not Meeting Public Health Exercise Guidelines. Psycho-Oncology. 2005;14:979–991. [DOI] [PubMed] [Google Scholar]
- 37.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–386. [DOI] [PubMed] [Google Scholar]
- 38.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowakowski GS, Blum KA, Kahl BS, et al. Beyond RCHOP: A Blueprint for Diffuse Large B Cell Lymphoma Research. J Natl Cancer Inst. 2016;108(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


