Figure 6. Pharmacological inhibition of PTPN11 in mouse melanoma cells with NRAS Q61K induces tumor regression in part through regulation of GSK3 and cyclin D1.
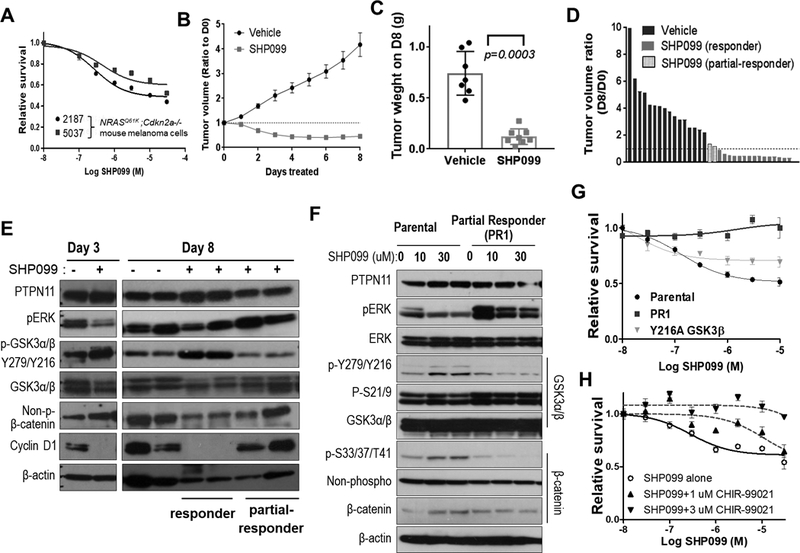
Survival analysis of 2187 and 5037 (mouse melanoma cells with Tyr-rtTA; tetO-NRAS Q61K; Cdkn2a (Ink4a/Arf) −/−) following treatment with increasing concentrations of SHP099 at 72 hours (A). Decreased tumor volume following treatment of established subcutaneous allograft 5037 tumors with SHP099 (PTPN11 inhibitor, 100 mg/kg; po qd) compared to treatment with vehicle alone. Data shown as mean tumor volume ratio to day 0 +/− SEM (B) and final tumor weight at harvest (p=0.0003; two-tailed t-test) on day 8 (C). Waterfall plot demonstrating ratio of D8 to D0 tumor volume for tumors treated with vehicle or SHP099. SHP099 treated tumor segregated into (red) responders and partial-responders (blue) with and without regression, respectively (D). Western analysis of 5037 subcutaneous tumors with (+) or without (–) SHP099 treatment for 3 or 8 days (E). Western analysis of parental 5037 and a cell line established from a partial responding tumor (PR1; blue in panel D) treated with 0, 10, or 30 uM SHP099 for 3 hrs (F). Cell survival was assessed at 72 hrs of post-treatment as the mean +/−SD survival (relative to DMSO or GSK3β inhibitor CHIR-99021 alone) (G&H). The response of 5037 with vector control or GSK3β-Y216A mutant and PR1 cells to SHP099 (G) and that of 5037 parental cells to increasing concentrations of SHP099 alone or in combination with 1μM or 3μM CHIR-99021 (H) were shown.
