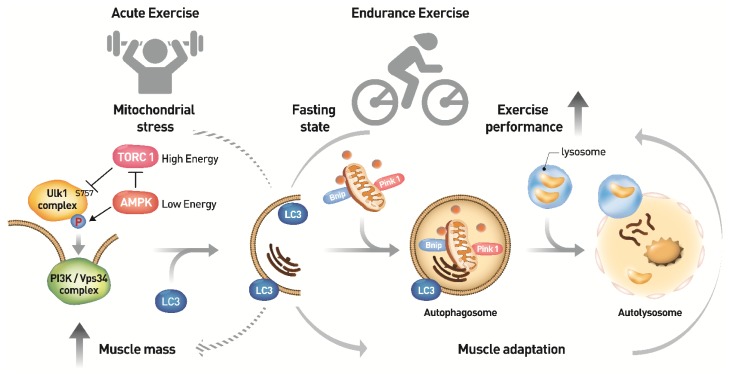
Fig. 2.
Molecular mechanism of autophagy induction by exercise in skeletal muscle. Nutrient depletion and low energy are well established autophagy inducers. Both mTORC1 inhibition and AMPK activation regulate the Ulk1 complex through a series of phosphorylation events leading to form a sequestering membrane called phagophore. LC3 conjugates to the sequestering membrane and controls the elongation of the phagophore, in which cytoplasmic organelles such as mitochondria and endoplasmic reticulum and aggregated proteins are occupied. PINK1-parkin complex and Bnip factors are required for removal of dysfuncional mitochondria. At the end of elongation, sequestering membrane closes and results in the formation of a double-membrane vesicle, autophagosome. Lysosomes ultimately fuse with autophagosomes releasing lysosomal hydrolases into the vesicle resulting in the degradation of its contents. An imperfect autophagy may contribute to the abnormal clearance of intracellular aggregates in skeletal muscle aging and lead to muscle loss. Endurance exercise-induced AMPK activation initiates autophagy induction. Upregulation of the early stage-initiating enzyme ATG7 by endurance exercise increases autophagy flux, thereby enhancing muscle performance. On the other hand, speculatively, the acute exercise might stimulate protein translation via mTORC1, thereby increasing muscle mass (broken arrow). However, the association between the acute exercise and autophagy formation needs further investigations in vivo.