Abstract
Bald thigh syndrome is a common hair loss disorder in sighthounds. Numerous possible causes, including environmental conditions, trauma, stress, endocrinopathies and genetic components have been proposed, but only endocrinopathies have been ruled out scientifically. The overall goal of our study was to identify the cause of bald thigh syndrome and the pathological changes associated with it. We approached this aim by comparing skin biopsies and hair shafts of affected and control dogs microscopically as well as by applying high-throughput technologies such as genomics, transcriptomics and proteomics. While the histology is rather unspecific in most cases, trichogram analysis and scanning electron microscopy revealed severe structural abnormalities in hair shafts of affected dogs. This finding is supported by the results of the transcriptomic and proteomic profiling where genes and proteins important for differentiation of the inner root sheath and the assembly of a proper hair shaft were downregulated. Transcriptome profiling revealed a downregulation of genes encoding 23 hair shaft keratins and 51 keratin associated proteins, as well as desmosomal cadherins and several actors of the BMP signaling pathway which is important for hair shaft differentiation. The lower expression of keratin 71 and desmocollin 2 on the mRNA level in skin biopsies corresponded with a decreased protein expression in the hair shafts of affected dogs. The genetic analysis revealed a missense variant in the IGFBP5 gene homozygous in all available Greyhounds and other sighthounds. Further research is required to clarify whether the IGFBP5 variant represents a predisposing genetic risk factor. We conclude from our results that structural defects in the hair shafts are the cause for this well-known disease and these defects are associated with a downregulation of genes and proteins essential for hair shaft formation. Our data add important knowledge to further understand the molecular mechanisms of HF morphogenesis and alopecia in dogs.
Introduction
One of the most characteristic features of hair follicles (HFs) is their self-renewal throughout the entire life of an individual to continuously produce new hair shafts (HSs). The self-renewal process is organized in the hair cycle during which the HF undergoes periodic stages of growth (anagen), regression (catagen), and quiescence (telogen) [1, 2]. The HS elongates during late anagen by the division of lineage-restricted matrix progenitors. These cells divide and migrate upward while differentiating to trichocytes. During differentiation, the nuclear function of the cells ceases, the non-keratin cell components break down and the synthesis of keratins and keratin-associated proteins (KAPs) increases. The precipitation of keratins and the loss of water finally results in terminal cornification, associated with hardening of the HS. During this process cell membranes of neighbouring trichocytes become more closely apposed and the number of cell junction complexes becomes higher, indicating an increased cellular adhesion and intercellular communication. At the level of the proximal end of the isthmus the mature HS detaches from the IRS (Adamson`s fringe) [3].
The individual trichocyte is composed of several macrofibrils, which are linked together by desmosomes containing several adhesion molecules such as desmoplakin and desmoglein. Macrofibrils in turn are composed of numerous intermediate filaments (IFs) embedded in an amorphous matrix composed of KAPs. An extensive network of inter- and intramolecular protein-protein crosslinks the IFs to the KAPs. The IFs are formed by a complex interaction of acidic type I and basic type II keratins [4, 5]. While the IFs provide the HS with a high tensile strength the matrix is important for the toughness, pliability, and resistance against microbial attacks [6]. Chemically, the HS is composed of 65–90% proteins and, depending on the humidity, up to 32% water. In addition, it contains lipids, pigments, and metals [7]. The main proteins of the trichocytes are keratins and KAPs. Hair keratins differ from epithelial keratins in their high content in cysteine residues used for the formation of inter- and intra-molecular disulfide bonds during the keratinization process [8]. Different keratins are expressed in the different regions of the HS. In humans, the medulla and cortex contain twelve different hair keratins, whereas only six different keratins are expressed in the cuticle. Furthermore, a high number of epithelial keratins are expressed in the medulla [9–11]. KAPs are classified by their amino acid composition into high sulfur, ultra-high sulfur and tyrosine/glycine rich KAPs [12]. Like keratins, KAPs are differentially expressed within the HS. In human hair, up to 19 high sulphur, 24 ultra-high sulphur, and 14 high-glycine tyrosine KAPs have been found in the cortex, whereas 22, 34, and nine different KAPs, respectively have been found in sheep [12]. The KAPs are encoded by a large multigene family of which 92 protein coding genes are currently known in humans [13, 14].
In cross section, the HS is composed of three different layers: the cuticle, the cortex and the medulla. The cuticle is the outermost, protective layer and consists of several layers of flat, thin overlapping trichocytes that are arranged like shingles on a roof. The cuticle cells undergo flattening as they emerge from their progenitors in the bulb. The distance of the cuticle cells from each other and the morphology varies between dog breeds and accounts for the smoothness of the hair and the tendency to felt [4, 15]. The cuticle is affected by environmental conditions such as harsh weather conditions and brushing [16]. The cortex accounts for the major mass of the hair fiber and is important for the tensile properties of the HS. It is built of trichocytes that change in shape from spherical close to the bulb to highly elongated and aligned with the long axis of the HS in the more distal parts. The medulla is the innermost layer of the HS and consists of a column of large, loosely packed and vacuolated cells with a high nuclear–cytoplasmic ratio. The cells are horizontally oriented and randomly staggered. The medulla can be very prominent in some species with coarse hair or can be absent in fine animal and human scalp hairs [12, 15, 17].
Bald thigh syndrome (BTS) is a hair loss disorder seen in Greyhounds and other sighthound breeds into which the Greyhound has been introgressed such as Whippets, Galgo Español, and Magyar Agár. BTS is characterized by bilateral hair loss on the caudal and lateral thighs, but alopecia may extend to the distal hind legs, the ventral abdomen and the chest. In some cases even the ventral neck is involved. Dogs of any age and sex may be affected. No scientific information is available under which circumstances the hair loss occurs but anecdotal information received from owners, breeders and internet forums indicate that the hair loss mostly starts when dogs are adult. It is frequently reported that hair loss begins when dogs start their training on the race track but it is also seen in dogs that are solely kept as companion dogs. Alopecia may wax and wane and no relation to stress or food can be made. It has been reported that up to 16% of the Greyhounds in long term training are affected [18]. The cause of BTS is unclear but numerous possible causes have been proposed. The alopecia has been associated with dogs rubbing against the sides of their crates, training methods, physical stress, diet, environmental conditions, estrous cycle and also an over-production of cortisol induced by a rigorous training program. In addition, a genetic component is suspected since BTS affects only Greyhounds and related breeds. In veterinary textbooks this syndrome is reported as pattern baldness [19, 20]. In the only scientific publication about this syndrome it was hypothesized that BTS is associated with hypothyroidism or hyperadrenocorticism. However, neither morphological changes in the adrenal glands, thyroid glands or the skin confirmed this hypothesis. Histological findings reported in this publication were not uniform in all Greyhounds with BTS. Common changes included dilatation of follicular infundibula, which often contained keratin and HSs and the presence of catagen HFs [21].
The overall goal of our study was to identify the cause of BTS and pathological changes associated with it. We addressed this by 1) histological evaluation of skin biopsies from Greyhounds with BTS in comparison to skin biopsies from haired Greyhounds, 2) investigation of the HS structure using trichograms and scanning electron microscopy of affected and control Greyhounds and Whippets, 3) transcriptome analysis of skin biopsies from affected and control Greyhounds to identify differences in gene expression in order to gain insight into the molecular mechanisms which may be involved in the pathogenesis of BTS, 4) assessment of the protein composition of HSs of affected and control dogs, and 5) comparison of whole genome sequence data from affected and unaffected Greyhounds in comparison to genome sequence data of dogs from other breeds that are not predisposed to BTS.
Material and methods
Ethics statement
All hair samples, EDTA blood and skin biopsies were taken with informed owner consent and with the permission of the cantonal animal welfare committee, Switzerland (permission number BE38/17). Permission of the cantonal animal welfare committee was given prospectively and comprises the intravenous withdrawal of blood and taking skin biopsies in dogs which have been identified by veterinarians to be suitable for the study. Biopsies were taken under local anesthesia using either lidocaine 2% or ropivacain 2%. In a few cases sedation was necessary. The dogs were sedated after 5 hours of fasting with medetomidine (5-10mcg/kg IM) and antagonised intramuscularely (IM) with the same volume of atepamizole as was given with medetomidine.
Skin biopsies
Skin biopsies were taken from seven Greyhounds with BTS, six control Greyhounds and one Magyar Agar. All dogs were privately owned and kept as pets. In addition, four archival biopsies from Greyhounds with BTS and six biopsies from control Greyhounds that were submitted to the University of Bern for routine diagnostic purposes were evaluated histologically. The diagnosis BTS was based on the clinical phenotype of the dogs by a board certified veterinary dermatologist (SR). Endocrinopathies were excluded by the absence of systemic signs and appropriate laboratory tests. From each dog two 6 mm punch biopsies were taken from the caudal thigh. In dogs with BTS the biopsies were taken in the alopecic area. From each dog one biopsy was immediately put in RNAlater (76106; Qiagen) and frozen at -80°C prior to RNA extraction. The other biopsy was fixed in 10% buffered formalin, processed routinely and stained with hematoxylin and eosin prior to the histological evaluation (S1 Table).
Histological analysis
We evaluated skin biopsies sampled for this study and archival biopsies from 11 Greyhounds with BTS and 13 controls (12 Greyhounds, 1 Magyar Agar), respectively. The formalin fixed biopsies were embedded in paraffin, cut as 4 μm section and stained with hematoxylin and eosin using standard procedures. The histological evaluation was performed blinded by two experienced dermatopathologists (DJW and MMW) and special attention was payed to the density, size and cycle stages of the HFs and the diameter of the HSs.
Trichogram analysis
Trichograms were assessed from dogs with BTS (Greyhounds, n = 6; Whippets, n = 10) and controls dogs (Greyhounds, n = 5; Whippets n = 12; Magyar Agar, n = 1) (S1 and S2 Tables).
To analyze HS quality, 10–70 HSs were plucked with a mosquito clamp from the border of the alopecic thigh and the haired back of affected dogs. In the alopecic areas only very few HSs were visible and these were plucked as well. A similar number of hairs were also plucked from the haired thigh and the back of control dogs. The hairs were placed on a glass slide, cleaned with two drops of chloral-lactophenol, covered with a cover slip and examined with a 200x magnification under the microscope. The hair cycle stage and the quality of the HSs were recorded.
Statistics were performed by assessing first normality of the data with the Shapiro test. Thereafter, the Wilcoxon signed rank test was used to assess the difference in the percentage of fractured HSs plucked from 1) the thighs of affected vs thighs of control dogs, 2) the back of affected dogs vs the back of control dogs, 3) the thighs of affected dogs vs the back of control dogs and 4) the back of affected dogs vs the thighs of control dogs. The paired samples Wilcoxon test was used to compare the percentage of fractured HSs plucked from 1) the thigh vs the back of affected dogs and 2) the thigh vs the back of control dogs. p values < 0.01 were regarded as statistically significant. Analyses were carried out in R language and environment for statistical computing (R version 3.5.1).
Scanning electron microscopy
To further assess the structure of the HSs scanning electron microscopy (SEM) was performed from the plucked hairs of three dogs with BTS and four unaffected controls (S1 Table). Hairs were plucked using the same method as described for trichograms. A total of 35 hairs from the back and thigh from control dogs and 29 hairs from the same locations from dogs with BTS were examined. Prior to scanning four to six hairs per location were placed in their total length on 25 mm wide aluminum stubs. The hair samples were coated with gold using the EM ACE600 Sputter (Leica) and scanned with the scanning electron microscope Helios NanoLab 650 SEM (FEI, now Fisher Scientific). From each sample digital images were taken in different magnifications.
RNA extraction and transcriptome sequencing (RNA-seq)
RNA for transcriptome sequencing was extracted from skin biopsies from the thigh of five dogs with BTS and seven control dogs (S1 Table). RNA extraction, library preparation and RNA-seq were performed as described in the detailed method description in S1 Text and a previous study [22]. The data are available in the European Nucleotide Archive (ENA), study accession PRJEB21761 and sample accessions SAMEA104393642-SAMEA104393658. (http://www.ebi.ac.uk/ena/data/view/PRJEB21761).
Mapping to reference genome and differential gene expression analysis
All reads that passed quality control were mapped to the dog genome reference (Can.Fam3.1) by STAR aligner version 2.5.3a [23] as described in our previous study [22]. The read abundance was calculated using HTseq and a gff3 file (version 104) obtained from NCBI canFam3.1 annotation release [24]. We used the DESeq2 package [23] to read the HTseq count data and filter for low/non-expressed genes. DESeq2 applies a generalized linear model (GLM) to the normalized count data assuming a negative binomial distribution. Read counts for each gene were fit to a GLM with 2 factor design model (~sex + condition) where condition was the factor of interest with two states: control and BTS, and sex factor was used to control for the effect of sex. Transcripts were considered to be differentially expressed with a Benjamini and Hochberg false discovery rate (FDR) of < 0.05. A detailed method description is available in S1 Text.
Proteomic analysis
Proteomic analysis was performed on fractured HSs of four dogs with BTS (Greyhounds, n = 3, Whippet, n = 1) and intact telogen HSs of four control dogs (Greyhounds, n = 3, Whippet = n = 1) plucked on the thighs (S1 Table). From each dog 0.25–0.29 mg of hair was cut in small pieces and washed successively three times with 50mM acetic acid and 20% ethanol before protein extraction as described [25] with some changes (see S1 Text). Extracted proteins were separated by SDS-PAGE and in-gel digested for nano liquid chromatography tandem mass spectrometry (nLC-MS/MS). Mass spectrometry data was processed with MaxQuant/Andromeda (version 1.5.4.1) to identify proteins at a 1% FDR and relatively quantify expression differences between control and BTS dog samples at a 5% FDR. Identical gel material, but without any protein sample loaded and processed in parallel, in order to assess keratin contaminations from the laboratory environment (S1 Text). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD012371.
Whole genome sequencing and variant calling
We sequenced the whole genome of two affected and two normal haired Greyhounds (S1 Table) to investigate the underlying genetic etiology. For each dog, an Illumina PCR-free TruSeq fragment library with ~450–500 bp insert size was prepared. We collected ~260–337 million 2 x 150 bp paired-end reads on a NovaSeq 6000 instrument. The reads were mapped and variants were called using BWA-GATK best practices workflow with the following versions of the tools—BWA (version 0.7.15), samtools (version 1.9), picard tools (version 1.8), and GATK (version 3.6)[26–28]. The functional effects of the called variants were predicted using SnpEFF software (version 4.3T) [29] together with the NCBI annotation release 104 on CanFam 3.1. The sequence data were deposited under the study accession PRJEB16012 and sample accessions SAMEA4867932, SAMEA4848711, SAMEA4867933, and SAMEA4848712, at the European Nucleotide Archive. Additionally, we used 355 additional whole genome sequences as controls, which were either publicly available [30], produced during other projects of our group or contributed by members of the Dog Biomedical Variant Database Consortium; ENA study and sample accession numbers are available in S12 Table and a detailed method description can be found in S1 Text.
Results
Histological findings
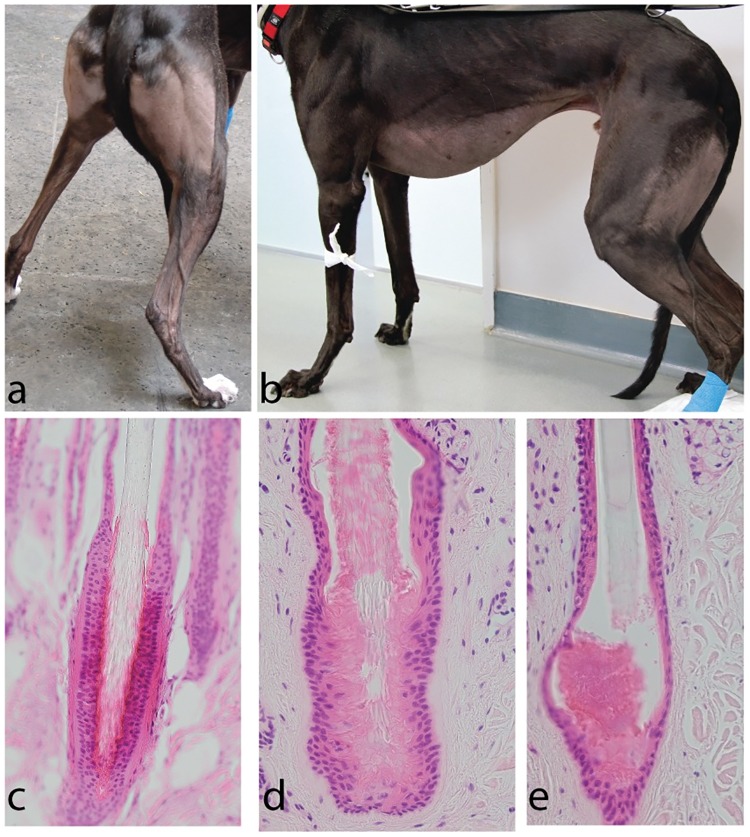
Histological findings were similar in all biopsies investigated and no clear difference between biopsies taken from alopecic skin and haired skin could be observed. In all biopsies, no matter if they were taken from alopecic skin of dogs with BTS or from haired skin of control dogs, HFs were present and of similar size. The follicular stage was not determinable in about 40% of the follicles due to the orientation of the biopsies, similar to previously published results in healthy dogs [31]. In the follicles where the cycle stage could be assessed, the anagen:telogen ratio was about 1:1 in both groups. Multiple HSs were present in the majority of the slightly dilated infundibula which contained otherwise a mildly increased amount of infundibular keratin in both groups. The diameters of the HSs did not differ between affected and control dogs. In one biopsy of one of the affected dogs one telogen HF was present which had an increased amount of trichilemmal keratin at the proximal end of the follicle and the more distal part of the HS was not anchored by trichilemmal keratin as is usually seen in club hairs (Fig 1d). In another biopsy of an affected dog the HS in one telogen HF was broken horizontally and not anchored by trichilemmal keratin as just described whereas the base of the HF was filled with abundant keratin (Fig 1e). The dermis was rather thin in both groups, which hampered correct embedding.
Fig 1. Clinical and histological phenotype of Greyhounds with BTS.
(a) Affected dog with alopecia on the caudal and lateral thighs extending below the lateral hock area; (b) Affected dog with alopecia on the caudal and lateral thighs, the lateral hind leg down to the hock, the ventral abdomen and the ventral chest; (c) Normal telogen HF; (d) Telogen HF with an increased amount of trichilemmal keratin at the proximal end of the follicle. The more distal part of the HS is not anchored by trichilemmal keratin; (e) Horizontally broken telogen HF, which is not anchored by trichilemmal keratin and abundant keratin at the base of the follicle.
Trichogram
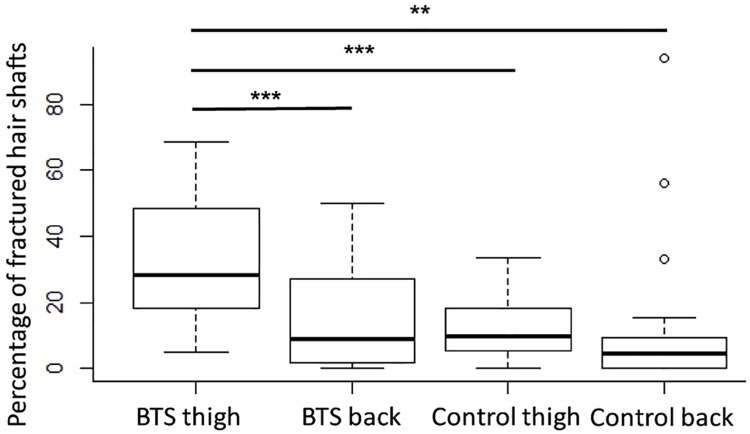
Trichogram analysis revealed a significantly higher number of HSs fractured on the proximal endin hairs plucked from the thigh of dogs with BTS. The number of fractured HSs was higher than from the back of dogs with BTS and from both locations in control dogs (S2 Table, Fig 2). However, clean transverse fractures at the proximal ends of the shafts were equally present on the back in both groups and on the thighs in control dogs (S2 Table, Fig 2). Unexpectedly, we found in two control dogs (C4 and C8) fractures in more than 50% of the plucked hairs from the back; visible as outliers in the boxplot. Other HS abnormalities, besides small melanin aggregates, depending on the colour of the dogs, were not observed in the trichograms.
Fig 2. Boxplots illustrating the percentage of fractured hair shafts plucked from the thigh and back of affected and control dogs.
The box includes the median of the data and the box edges are the 25th and 75th percentiles. The vertical size of the boxes displays the interquartile range (IQR); the whiskers represent the minimum and maximum values that do not exceed 1.5 x IQR from the median. ** p < 0.01 *** p < 0.001.
Scanning electron microscopy
SEM revealed several structural defects in HSs from Greyhounds with BTS and control dogs. The most frequently observed abnormality in control and affected dogs was a clean transverse fracture (trichoschisis) in the proximal part of the hair fiber (17/64 hairs). The percentage of fractured HSs was higher in Greyhounds with BTS (34%) as compared to controls (20%). The second common abnormality was a central longitudinal splitting (central trichoptilosis) of hair fibers. The splits varied in length and were between 50μm and 1.5mm long (7/64 hairs). The percentage was higher in dogs with BTS (17%) in comparison to 5.7% in controls. Rare findings were longitudinal grooves and an irregular outer contour of the HSs with fused cuticular cells (Fig 3) [32].
Fig 3. Structural abnormalities in hair fibers of Greyhounds identified by scanning electron microscopy.
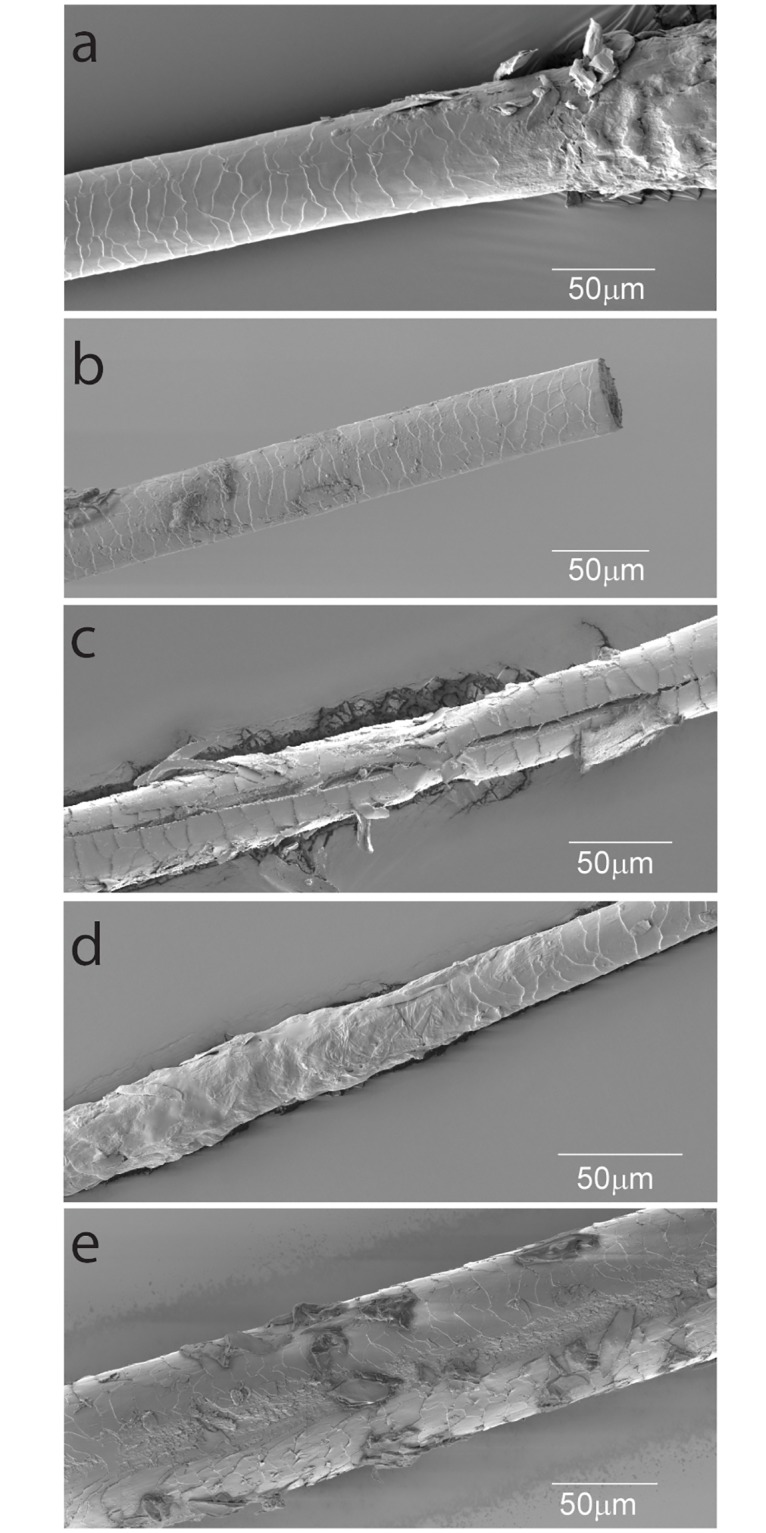
(a) Intact telogen hair root of a control Greyhound, (b-d) abnormal HSs from Greyhounds with BTS (b) Clean transverse fracture (trichoschisis); (c) Central longitudinal splitting (central trichoptilosis). (d) Irregular surface of the cuticle; (e) Longitudinal grooves.
Differentially expressed genes
Principal component analysis of the RNA-seq data revealed that BTS samples were successfully separated from control samples. In addition, clustering based on sex was found within the control group (S1 Fig). Cook’s distance revealed one outlier, which was excluded from the analysis (sample C3). It was a male Greyhound classified as unaffected control dog within our project. Histopathological re-evaluation of the skin biopsies revealed a low grade superficial perivascular and lymphocytic dermatitis, which could explain the large Cook’s distance and justified the exclusion of that sample. After filtering for low/non-expressed genes we analyzed a total of 22503 genes. We considered genes with an FDR of less than 0.05 as differentially expressed and discovered 442 differentially expressed genes. Of those, 74 genes (17%) were upregulated and 368 genes (83%) were downregulated in BTS affected dogs (S3 Table).
Numerous downregulated genes encode structural proteins such as keratins (23 genes) and KAPs (51 genes) of the HS and the IRS cuticle (S4 and S5 Tables).
Additionally, we identified 14 downregulated genes involved in HF and HS differentiation and variants in some of these genes are associated with HS disorders. Furthermore, genes encoding members of the BMP signaling pathway, BMP2 and BMP4, were downregulated as well as some of their downstream acting genes (FOXN1, HOXC13 and MSX2), which are known to play a role in HS formation (S6 Table).
Proteomic analysis
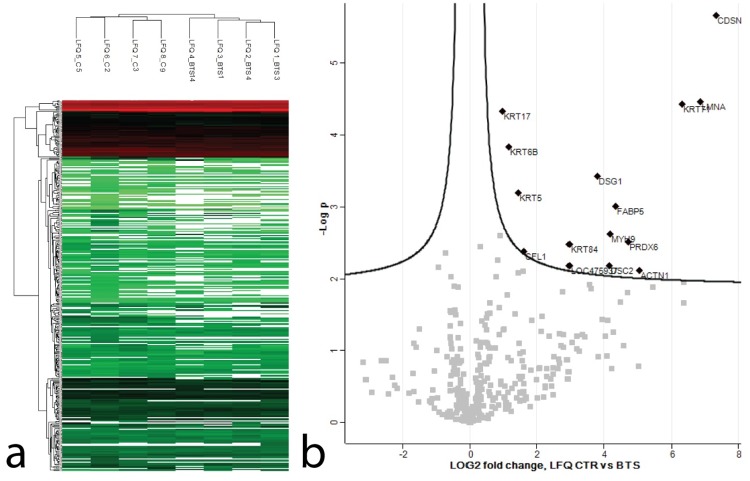
To confirm the results of the transcriptome analysis from whole skin and to further elucidate the structure of defect HSs of dogs with BTS, we performed proteomic profiling using a nano-scale liquid chromatographic tandem mass spectrometry (nLC-MS/MS) approach. Our aim was to identify differences in protein expression of fractured HSs from dogs with BTS (3 Greyhounds, 1 Whippet) as compared to intact telogen HSs from control dogs (3 Greyhounds, 1 Whippet). A total of 353 proteins were detected in HSs of dogs (S7 Table). Of those, 23 were identified as possible contaminants and 27 proteins were only identified by a modification site. After performing a Student’s T-test on log2-transformed label free quantitation (LFQ) values 15 significantly differentially expressed proteins between the control and BTS group with a log2 fold change above one were identified (Fig 4, S8 Table).
Fig 4. Canine hair proteins identified by nLC-MS/MS.
(a) Hierarchical clustering of LFQ protein intensities. Highly abundant proteins are shown in red, low abundances in green, intermediate values in different shades of red and green and missing protein values are displayed in white. BTS affected dogs and control dogs form two distinct clusters. (b) Volcano plot representing the results of the T-test performed on LFQ intensities of control vs BTS. Differentially expressed proteins with a FDR < 0.05 and a log2 fold change cut-off value of 1 are labeled.
Genetic analysis
To investigate possible genetic risk factors for BTS, we used whole genome sequencing data from two BTS affected and two unaffected Greyhounds as well as 355 dog genomes from diverse breeds. Applying a hard-filtering approach, we considered a recessive and a dominant mode of inheritance as well as a fixed risk allele in the Greyhound population (S1 Text). In the model of fully penetrant autosomal recessive mode of inheritance, 5 variants predicted to be protein-changing were exclusively found in homozygous state in the affected Greyhounds (S9 Table). One of them, a missense variant in the CYP26C1 gene encoding an enzyme involved in the regulation of retinoic acid levels, was specific to Greyhounds in our sample. Filtering for variants consistent with a dominant mode of inheritance resulted in 64 protein-changing variants (S10 Table). These variants included a missense variant in the BRD8 gene, encoding a thyroid-hormone receptor interacting protein. In both recessive and dominant scenarios, none of the protein changing variants was located in an obvious functional candidate gene or one of the genes that showed strong differential expression. Searching for variants that were present in the homozygous state in all available Greyhounds but not in any non-sighthound breed resulted in only one protein-changing variant (S11 Table), a missense variant in the IGFBP5 gene; XM_847792.4:c.424C>T, p.(Arg142Cys). This variant was also present in a homozygous state in a Whippet, a Scottish Deerhound and three Sloughis, and present in a heterozygous state in a Saluki. We had no reliable information regarding a possible BTS phenotype in the other sequenced sighthounds. The twelve Greyhound transcriptomes obtained in this study also were from dogs that were homozygous for the alternate T-allele at this variant.
Discussion
In our study we identified structural defects in the HSs as underlying cause for BTS in Greyhounds and related breeds. Transverse fractures and other structural defects in the proximal parts of the HSs have been discovered by trichograms and SEM. These findings are supported by the histological analysis and the results of the transcriptomic and proteomic profiling where genes and proteins important for differentiation of the IRS and the assembly of a proper HS were downregulated.
Our results contradict the description in a veterinary textbook in which BTS is reported as pattern baldness [33]. The histological characteristic of this type of alopecia is the miniaturization of HFs resulting in thin to minuscule HSs, which cannot be appreciated in skin biopsies from dogs with BTS when compared to biopsies from the same body location of haired control dogs. Histologically, we saw in both affected and control dogs mildly dilated HF infundibula with numerous HSs of a similar diameter in the lumen. In addition, we found a similar percentage of anagen and telogen follicles, as has been described in another study where dogs with a short and fine hair coat have been histologically evaluated [34]. In the only so far existing histological study of BTS a dilatation of follicular infundibula filled with keratin and hair has also been described [21]. However, in this study the authors did not compare skin biopsies of alopecic Greyhounds with control dogs of other breeds. Thus, we assume that a mild dilation of the infundibulum is a breed-specific feature in the caudal thighs of Greyhounds and other sighthound breeds. In addition, the presence of normal sized HSs within the infundibulum does neither support a hair cycle arrest, in which HSs are not produced any longer, nor a pattern baldness in which the HSs should be thinner than in control dogs.
Using light and SEM we found a significantly higher percentage of HS defects on the caudal thighs of the affected Greyhounds and Whippets as compared to the back of the same dogs and the back and the thighs of control dogs. HS defects can be attributed to mechanical, physical, and chemical injury or may have an underlying inherited cause [35]. Damage caused by environmental insults is mostly seen in the exposed distal parts of the HSs, whereas inherited defects can also occur in the more proximal parts of the HS which are protected within the HF [36]. The HS fractures in our study group were exclusively seen in the proximal part close to the hair root, suggesting an inherited defect. The fact that the percentage of fractured HSs was significantly higher on the thighs of affected dogs as compared to the back of the same dogs might be explained by different hair types on different body locations. In addition, the severity of structural defects may change due to yet unknown circumstances, which might explain that BTS is waxing and waning in some dogs and that some dogs recover completely.
The most frequently observed defects were clean transverse fractures (central trichoschisis) and central longitudinal splitting (central trichoptilosis). In addition, we found HSs with longitudinal grooves. These HS defects are known in humans and causative genetic variations have been identified in some of them [36–39]. Furthermore, we identified HSs with an irregular outer contour and fused cuticular cells, a structural defect which has not been described so far. Central trichoptilosis and HSs with longitudinal grooves have been described in humans in association with ectodermal dysplasia [40]. Ectodermal dysplasia has been defined as an abnormal development of at least two different tissues or organs of ectodermal origin [41–45]. Since no other tissue defect has been identified in sighthounds with BTS the HS defects cannot be attributed to ectodermal dysplasia in the strict meaning. However, several other conditions in humans exist in which the HS is the only ectodermal appendage affected and in some of them an underlying genetic defect has been identified. Described alterations of the HS associated with hypotrichosis or alopecia range from thin, brittle, or coarse HSs to severe structural defects within the HSs [36, 41, 43, 44]. The far best described HS defect in humans is monilethrix. In this nonsyndromic hair disorder the HS has intermittent constrictions separating elliptical nodes of normal thickness. Most of the cases of monilethrix are caused by variants in keratin genes, but it is also described with variants in the desmoglein 4 gene (DSG4). [41, 44, 46–48]. Trichothiodystrophy (TTD) is another heterogeneous group of autosomal recessive disorders in which clean transverse fractures of the HSs, similar to those seen in our study are the morphological hallmark. Furthermore, an irregular hair surface, HS diameter and a decreased cuticle layer may be present. The underlying cause of TTD is a defective synthesis of high-sulfur matrix proteins (reviewed in [36, 39, 41]. In all so far described HS disorders in humans the HSs break easily with trauma caused by environmental insults or mechanical stress. Interestingly, also in humans hair loss may occur periodically and intermittent hair loss associated with infections has been described. As suggested before, trauma or subclinical infections may also explain the waxing and waning of the alopecia seen in dogs with BTS.
In order to investigate the molecular pathomechanisms involved in BTS we performed RNA-seq of whole skin biopsies to define differences in gene expression levels between affected and control dogs. A total of 23 genes encoding HF and HS specific keratins and 51 genes encoding KAPs was downregulated in the skin of dogs with BTS (S4 and S5 Tables). Both keratins and KAPs are the major proteins of the trichocytes and essential for a mechanically stable and compact hair fiber [12, 49]. Thus, these results suggest that the HS defects identified in BTS are the consequence of the downregulation of several genes encoding for proteins which are crucial for the mechanical stability and integrity of HS [3]. Interestingly, the keratin genes KRT81, KRT83, KRT86, as well as the desmosomal cadherin-coding gene DSG4, were downregulated in dogs with BTS. Variants within these genes have been identified in monilethrix but the expression has not been investigated on the mRNA level [47, 48, 50]. Vice versa genetic analysis in our cases did not reveal variants within these genes. The keratin gene family is largely, but not perfectly conserved between humans and dogs [51].
Beside the downregulation of keratins and KAPs we observed downregulation of 14 other genes involved in HS differentiation and assembly of the HS (S6 Table). Amongst those, we identified a significant downregulation of BMP2 and BMP4 as well as the downstream acting regulators of HS differentiation MSX2, FOXN1 and HOXC13 in skin biopsies of dogs with BTS. It has been shown in mice that ablation of Bmp signaling downregulates the expression of several transcription factors such as Msx2, Fox1 and Hoxc13 resulting in a reduced expression of acidic hair keratins and trichohyalin leading to a severely impaired HS differentiation [52]. Other downregulated genes, namely PADI3 and TCHH are associated with uncombable hair syndrome in humans [53] and S100A3 has been connected with an age-dependent damage of the hair cuticle [54, 55]. Furthermore, it is well known that desmosomal cadherins such as the downregulated DSC2 and DSG4 are crucial to crosslink the IFs and the KAPs and are thus essential for a mechanically stable and compact hair fiber [8].
To further elucidate the origin of the HS defects, we performed proteomic analysis of fractured HSs from dogs with BTS and intact telogen HSs from control dogs. Proteomic analysis revealed differential protein expression of 15 proteins. Among others, we found a decreased protein expression of keratin 71 (KRT71), desmoglein 1 (DSG1), corneodesmosin (CDSN), and desmocollin 2 (DSC2). In line with the downregulation of KRT71 on the mRNA level is the decreased protein expression of KRT71 (Table 1). This suggests that KRT71 plays an important role in the pathomechanism of BTS, although no sequence variant in the gene has been identified in dogs with BTS. In mice and humans KRT71 protein is expressed in the IRS [56, 57], but the localization of KRT71 expression has not been shown in dogs yet. KRT71 is crucial for hair texture and different variants in the KRT71 gene result in wooly or curly hair phenotypes seen in caracul mice, rex rats, Devon Rex cats and many curly coated dogs [58–60]. The autosomal dominant Rex variant in rats leads to hair loss in the homozygous state [61]. In reduced coat 3 (Rco3) mice carrying an autosomal recessive variant in Krt71 progressive alopecia develops due to alterations in the keratinization of the IRS and subsequent structural defects in the HSs [62]. In the hairless Sphynx cat a variant in KRT71 in a homozygous state or compound heterozygous with the curly (re) allele of the closely related Devon Rex cats leads to abnormalities of IRS and misshapen HSs [63]. Furthermore, a missense variant in KRT71 causes the autosomal dominant wooly hair and hypotrichosis phenotype in humans and in cells transfected with the mutant KRT71 protein a disruption of IF formation was observed [64]. Since KRT71 is known to be expressed in the IRS in mice and humans, and the expression in HSs has not been described yet, further investigation of KRT71 protein expression in the HSs of dogs is needed.
Table 1. Differential gene and protein expression in hair shafts and skin of dogs with BTS vs controls.
| Protein expression | Gene expression | |||
|---|---|---|---|---|
| Gene | FDR | log2FC BTS vs Ctrl | FDR | log2FC BTS vs Ctrl |
| KRT71 | 2.13E-02 | -6.32 | 1.07E-10 | -1.51 |
| DSC2 | 2.90E-02 | -4.15 | 3.01E-02 | -0.52 |
Differential protein expression was investigated by proteomic profiling of fractured hair shafts from dogs with BTS vs intact telogen hair shafts from controls. Differential gene expression was studied using RNA-seq of whole skin biopsies from dogs with BTS and controls.
We further found a downregulation of DSC2 on the mRNA level in skin biopsies and a decreased protein expression in the defect HSs of dogs with BTS (Table 1).
Dsc2 is expressed in the medulla of HSs in mice, specifically between the vertically assembled medulla cells and between cortex and medulla cells and it is assumed that DSC2 plays an essential role in the assembly of the hair medulla [65, 66]. Interestingly, in mice the expression of Dsc2 in medulla has been associated with the expression of Foxn1, a transcription factor downstream of Bmp, which is also downregulated in dogs with BTS [67]. Foxn1 deficient mice (nude mice) display HS defects which lead to a fragmentation of HSs and subsequent alopecia in mutant mice. The reduced transcription of Dsc2 in HSs and low abundance of Dsc2 protein in medulla cells adjacent to cortex cells as a consequence of Foxn1 deficiency result in improper assembly of medulla cells and therefore may be the reason for fragile HSs in nude mice [66].
Two other proteins of interest which were decreased in fractured HSs are CDSN and DSG1. CDSN is expressed in the IRS and in squamous epithelium of skin and variants in CDSN in humans are associated with hypotrichosis simplex of the scalp. Patients with hypotrichosis simplex develop progressive hair loss starting in late childhood. As possible pathomechanism for the alopecia, the authors discuss a loss of cohesion within the IRS or protein aggregates of CDSN [68]. DSG1 is an important protein of desmosomes which anchor IFs within their matrix and assemble macrofibris [3, 69]. The reduced protein may impair tissue integrity of HSs and assembly of cortex and medulla cells and thus result in defect HSs in dogs with BTS.
A limitation of our study is the fact that transcriptional and proteomic profiling has not always been performed with the same dogs (S1 Table) and thus comparison of the results may have some weaknesses. However, the finding that even in whole skin biopsies genes coding for HS proteins are significantly downregulated in biopsies from dogs with BTS and that two of these are even differentially expressed on the protein level strongly suggests a correlation and biological significance. We are also aware that protein expression might be influenced by the missing very proximal part of the fractured HS, which is present in the intact telogen HSs and we face the risk that the lower expression of some proteins may be interpreted wrongly.
In our genetic study we did not identify a compelling candidate genetic variant that co-segregated with the BTS phenotype. This is most likely due to a complex mode of inheritance, which would not have been tractable with our simple hard filtering approach in only two cases and two controls. However, we found a missense variant in the IGFBP5 gene that was apparently fixed in the homozygous state in all available Greyhounds and some other sighthounds and absent or rare in other breeds. Insulin-like growth factor binding proteins (IGFBPs) are secreted proteins that are able to bind and antagonistically modulate effects of insulin-like growth factors. IGFBP5 has an influence on numerous biological processes, but mainly its potential to enhance or suppress cell proliferation has been investigated. Igfbp5 mRNA is expressed in the dermal papilla of murine anagen HFs as well as in dermal cells and the subcutis. The expression of Igfbp5 in the dermal papilla decreases at the beginning of catagen, while the expression in the dermis is maintained during the resting phase of the hair cycle [70]. Furthermore, Igfbp5 expression correlated with the bent structure of zigzag hair, one of the four hair types of mouse pelage. In transgenic mice, ectopic Igfbp5 expression led to thin and curved hair [71]. There is a lack of literature about IGFBP5 expression in dogs, however, given the knowledge in mice, it is likely that IGFBP5 also has an influence on HS differentiation in dogs and we cannot exclude that the expression pattern of IGFBP5 in the HFs of the thighs and the ventral aspects of the dogs differs from the HFs of the back. Whether the identified genetic variant has an impact on protein function and whether it represents a predisposing risk factor for BTS remains however to be determined.
Taken together, we have shown that BTS is caused by structural HS defects which are associated with the downregulation of genes and proteins essential for HS differentiation and HS assembly. The underlying genetic defect has not yet been identified and we suggest a complex mode of inheritance. Our data add important knowledge to further understand the molecular mechanisms of hair shaft formation and alopecia in dogs.
Supporting information
(DOCX)
BTS, bald thigh syndrome; C, control; m, male; mc, male castrated; f, female; fc, female castrated; SEM, scanning electron microscopy; WGS, whole genome sequencing.
(XLSX)
* Hairs were plucked from the hypotrichotic area on the border alopecia/haired.
(XLSX)
ID, gene identifier of the Entrez Gene database of NCBI; BaseMean, mean of normalized read counts across all samples; LfcSE, standard error of the log2 Fold Change; Stat, the log2 fold change divided by lfcSE; FDR, false discovery rate, refers to Benjamini-Hochberg adjusted p- value.
(XLSX)
ID, gene identifier of the Entrez Gene database of NCBI; Log2FC, log2 fold change; FDR, False discovery rate, refers to Benjamini-Hochberg adjusted p- value. Keratin expression in humans and sheep as reviewed in Harland, D.P. and Plowman, J.E. [3].
(XLSX)
ID, gene identifier of the Entrez Gene database of NCBI.; Log2FC, log2 fold change; FDR, false discovery rate, refers to Benjamini-Hochberg adjusted p- value.
(XLSX)
ID, gene identifier of the Entrez Gene database of NCBI; Log2FC, log2 fold change; FDR, false discovery rate, refers to Benjamini-Hochberg adjusted p- value; IRS, inner root sheath; HF, hair follicle; ORS, outer root sheath; DP, dermal papilla. [50, 52–55, 65, 66, 72–83].
(XLSX)
iBAQ, intensity based absolute quantification; LFQ, label free quantitation; top3, median normalized intensities of the three most intense peptides.
(XLSX)
Missing protein intensities were replaced sample wise by imputing a random number from the low end of the log2-transformed LFQ distribution; test differences were calculated on the median LFQ intensities (log2 transformed) and p values (-log) were corrected for multiple testing by a permutation based approach to estimate a 5% false discovery rate (q values); significant test results are marked by “+” in the “significant C vs BTS” column, if a protein in the control group is significantly higher expressed than in BTS and gene names of significantly differentially expressed proteins are highlighted in bold characters.
(XLSX)
0 stands for the wild type allele, 1 for the variant allele. Dog GY432 was excluded due to unknown phenotype.
(XLSX)
0 stands for the wild type allele, 1 for the variant allele. Dog GY432 was excluded due to unknown phenotype.
(XLSX)
0 stands for the wild type allele, 1 for the variant allele.
(XLSX)
(XLSX)
Samples are plotted across the two most variable components (PC1 and PC2) and sample clustering is based on condition.
(TIF)
Acknowledgments
The authors would like to thank dog owners, breeders and veterinarians for their support of this study. We thank Muriel Fragnière, Sabrina Schenk and Nathalie Besuchet-Schmutz of the Next Generation Sequencing Platform of the University of Bern for performing sequencing experiments and the Interfaculty Bioinformatics Unit and the HPC cluster UBELIX (http://www.id.unibe.ch/hpc) at the University of Bern for providing computational infrastructure. We also would like to thank Prof. Michael Stoffel for introducing us to the Keyence VHX-6000 digital microscope and to allow us to use this device.
Data Availability
Data are available from the European Nucleotide Archive and GenBank (accession no. PRJEB21761 and PRJEB16012) as well as from ProteomeXchange via the PRIDE database (accession no. PXD012371). All other data are available in the paper and its Supporting Information files.
Funding Statement
This study was supported by a grant from the Swiss National Science Foundation (CRSII3_160738 / 1). The funders provided support in the form of the salary for one author (Magdalena Brunner) and consumables, but had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Silvia Rüfenacht, one of the coauthors, owns a private dermatological specialty clinic (Dermavet) and generates her income by seeing patients. She was involved in the planning of the study and collected the biopsy and trichogram samples. Furthermore she evaluated the trichogram samples. She did not receive any payment for this work. The specific roles of all authors are articulated in the ‘author contributions’ section.
References
- 1.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144(1):92–105. Epub 2011/01/11. 10.1016/j.cell.2010.11.049 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5(3):267–78. Epub 2009/08/12. 10.1016/j.stem.2009.06.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harland DP, Plowman JE. Development of Hair Fibres. Adv Exp Med Biol. 2018;1054:109–54. Epub 2018/05/26. 10.1007/978-981-10-8195-8_10 . [DOI] [PubMed] [Google Scholar]
- 4.Koehn H, Clerens S, Deb-Choudhury S, Morton JD, Dyer JM, Plowman JE. The Proteome of the Wool Cuticle. J Proteome Res. 2010;9:2920–8. 10.1021/pr901106m [DOI] [PubMed] [Google Scholar]
- 5.Deb-Choudhury S. Crosslinking Between Trichocyte Keratins and Keratin Associated Proteins. Adv Exp Med Biol. 2018;1054:173–83. Epub 2018/05/26. 10.1007/978-981-10-8195-8_12 . [DOI] [PubMed] [Google Scholar]
- 6.Fraser RDB, Parry DAD. Amino acid sequence homologies in the hard keratins of birds and reptiles, and their implications for molecular structure and physical properties. J Struct Biol. 2014;188(3):213–24. 10.1016/j.jsb.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 7.Velasco MVR, Dias TCdS, Freitas AZd, V ND Júnior, Pinto CASdO, Kaneko TM, et al. Hair fiber characteristics and methods to evaluate hair physical and mechanical properties. Braz J Pharm Sci. 2009;45:153–62. [Google Scholar]
- 8.Rogers GE. Hair follicle differentiation and regulation. Int J Dev Biol 2004;48:163–70 [DOI] [PubMed] [Google Scholar]
- 9.Langbein L, Rogers MA, Winter H, Praetzel S, Beckhaus U, Rackwitz HR, et al. The catalog of human hair keratins. I. Expression of the nine type I members in the hair follicle. J Biol Chem. 1999;274(28):19874–84. Epub 1999/07/03. . [DOI] [PubMed] [Google Scholar]
- 10.Langbein L, Rogers MA, Praetzel-Wunder S, Bockler D, Schirmacher P, Schweizer J. Novel type I hair keratins K39 and K40 are the last to be expressed in differentiation of the hair: completion of the human hair keratin catalog. J Investig Dermatol. 2007;127(6):1532–5. Epub 2007/02/16. 10.1038/sj.jid.5700734 . [DOI] [PubMed] [Google Scholar]
- 11.Langbein L, Rogers MA, Winter H, Praetzel S, Schweizer J. The catalog of human hair keratins: II. Expression of the six type II members in the hair follicle and the combined catalog of human type I and II keratins. J Biol Chem. 2001;276(37): 35123–32. 10.1074/jbc.M103305200 [DOI] [PubMed] [Google Scholar]
- 12.Rogers MA, Langbein L, Praetzel-Wunder S, Winter H, Schweizer J. Human hair keratin-associated proteins (KAPs). Int Rev Cytol. 2006;251:209–63. Epub 2006/08/31. 10.1016/S0074-7696(06)51006-X . [DOI] [PubMed] [Google Scholar]
- 13.HGNC Database [Internet]. European Molecular Biology Laboratory, European Bioinformatics Institute. 2018 [cited October 2018]. www.genenames.org.
- 14.Shimomura Y, Ito M. Human Hair Keratin-Associated Proteins. J Investig Dermatol Symp Proc. 2005;10(3):230–3. 10.1111/j.1087-0024.2005.10112.x [DOI] [PubMed] [Google Scholar]
- 15.Tumilowicz P, Goliszewska A, Arct J, Pytkowska K, Szczepanik M. Preliminary study of guard hair morphology in four dog breeds. Vet Dermatol. 2018. Epub 2018/06/20. 10.1111/vde.12656 . [DOI] [PubMed] [Google Scholar]
- 16.Garcia ML, Epps JA, Yare RS, Hunter LD. Normal cuticle-wear patterns in human hair. J Soc Cosmet Chem. 1978;29(3):155. [Google Scholar]
- 17.Langbein L, Yoshida H, Praetzel-Wunder S, Parry D, S J. The keratins of the human beard hair medulla: the riddle in the middle. J Investig Dermatol. 2010;130(1). 10.1038/jid.2009.192 [DOI] [PubMed] [Google Scholar]
- 18.Lord LK, Yaissle JE, Marin L, Couto CG. Results of a web-based health survey of retired racing Greyhounds. J Vet Intern Med. 2007;21(6):1243–50. Epub 2008/01/17. . [DOI] [PubMed] [Google Scholar]
- 19.Gross TL, Ihrke P.J., Walder E.J., Affolter V.K. Atrophic diseases of the adnexa In: Gross TL, Ihrke P.J., Walder E.J., Affolter V.K., editor. Skin Diseases of the Dog and Cat, Clinical and Histopathologic Diagnosis. 2nd ed Oxford: Blackwell publishing; 2005. p. 480–513. [Google Scholar]
- 20.Broek A. Muller & Kirk’s Small Animal Dermatology. William Miller Jr, Craig Griffin, Karen Campbell. Saunders-Elsevier, St Louis; 7th Edition, 2013, 938 p; IBSN: 978-1-4160-0028-0, £113. Veterinary Dermatology. 2013;24(5):559-. 10.1111/vde.12055 [DOI] [Google Scholar]
- 21.Schoning PR, Cowan LA. Bald thigh syndrome of Greyhound dogs: gross and microscopic findings. Vet Dermatol. 2000;11(1):49–51. 10.1046/j.1365-3164.2000.00175.x [DOI] [PubMed] [Google Scholar]
- 22.Brunner MAT, Jagannathan V, Waluk DP, Roosje P, Linek M, Panakova L, et al. Novel insights into the pathways regulating the canine hair cycle and their deregulation in alopecia X. PLoS One. 2017;12(10):e0186469 Epub 2017/10/25. 10.1371/journal.pone.0186469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love M, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders S, Pyl P, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–9. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong SY, Lee CC, Ashrafzadeh A, Junit SM, Abrahim N, Hashim OH. A High-Yield Two-Hour Protocol for Extraction of Human Hair Shaft Proteins. PLoS One. 2016;11(10):e0164993 10.1371/journal.pone.0164993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poplin R, Ruano-Rubio V, DePristo MA, Fennell TJ, Carneiro MO, Van der Auwera GA, et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv. 2018. 10.1101/201178 [DOI] [Google Scholar]
- 27.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43(1110):11.0.1–33. 10.1002/0471250953.bi1110s43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27(21):2987–93. Epub 2011/09/08. 10.1093/bioinformatics/btr509 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6(2):80–92. Epub 2012/04/01. 10.4161/fly.19695 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai B, Zhao W-M, Tang B-X, Wang Y-Q, Wang L, Zhang Z, et al. DoGSD: the dog and wolf genome SNP database. Nucleic Acids Res. 2015;43(Database issue):D777–D83. Epub 2014/11/17. 10.1093/nar/gku1174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muntener T, Doherr MG, Guscetti F, Suter MM, Welle MM. The canine hair cycle—a guide for the assessment of morphological and immunohistochemical criteria. Vet Dermatol. 2011;22(5):383–95. Epub 2011/03/16. 10.1111/j.1365-3164.2011.00963.x . [DOI] [PubMed] [Google Scholar]
- 32.Mallory SB. An Illustrated Dictionary of Dermatologic Syndromes. Second ed: CRC Press; 2006. [Google Scholar]
- 33.Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis.: Blackwell Science Ltd; 2008. [Google Scholar]
- 34.Muntener T, Schuepbach-Regula G, Frank L, Rufenacht S, Welle MM. Canine noninflammatory alopecia: a comprehensive evaluation of common and distinguishing histological characteristics. Vet Dermatol. 2012;23(3):206–e44. Epub 2012/05/12. 10.1111/j.1365-3164.2012.01049.x . [DOI] [PubMed] [Google Scholar]
- 35.Marsh J, Gray J, Tosti A. Healthy Hair: Springer International Publishing; 2015. [Google Scholar]
- 36.Itin PH, Fistarol SK. Hair shaft abnormalities—clues to diagnosis and treatment. Dermatology. 2005;211(1):63–71. Epub 2005/06/29. 10.1159/000085582 . [DOI] [PubMed] [Google Scholar]
- 37.Tosti A, Piraccini BM. Diagnosis and Treatment of Hair Disorders: An Evidence-Based Atlas. 1 ed: CRC Press; 2005. [Google Scholar]
- 38.Itin PH, Sarasin A, Pittelkow MR. Trichothiodystrophy: update on the sulfur-deficient brittle hair syndromes. J Am Acad Dermatol. 2001;44(6):891–920; quiz 1–4. Epub 2001/05/23. 10.1067/mjd.2001.114294 . [DOI] [PubMed] [Google Scholar]
- 39.Faghri S, Tamura D, Kraemer KH, Digiovanna JJ. Trichothiodystrophy: a systematic review of 112 published cases characterises a wide spectrum of clinical manifestations. J Med Genet. 2008;45(10):609–21. Epub 2008/06/25. 10.1136/jmg.2008.058743 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirano-Ali SA, Reed AM, Rowan BJ, Sorrells T, Williams JV, Pariser DM, et al. Scanning Electron Microscopic Hair Shaft Analysis in Ectodermal Dysplasia Syndromes. Pediatr Dermatol. 2015;32(6):836–44. 10.1111/pde.12674 [DOI] [PubMed] [Google Scholar]
- 41.Duverger O, Morasso MI. To grow or not to grow: Hair morphogenesis and human genetic hair disorders. Semin Cell Dev Biol. 2013;25–26:22–33. Epub 2013/12/24. 10.1016/j.semcdb.2013.12.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinheiro M, Freire-Maia N. Ectodermal dysplasias: a clinical classification and a causal review. Am J Med Genet. 1994;53(2):153–62. Epub 1994/11/01. 10.1002/ajmg.1320530207 . [DOI] [PubMed] [Google Scholar]
- 43.Shimomura Y, Christiano AM. Biology and genetics of hair. Annu Rev Genomics Hum Genet. 2010;11:109–32. 10.1146/annurev-genom-021610-131501 . [DOI] [PubMed] [Google Scholar]
- 44.Shimomura Y. Journey toward unraveling the molecular basis of hereditary hair disorders. J Dermatol Sci. 2016;84(3):232–8. Epub 2016/08/16. 10.1016/j.jdermsci.2016.08.006 . [DOI] [PubMed] [Google Scholar]
- 45.Freire-Maia N, Lisboa-Costa T, Pagnan NA. Ectodermal dysplasias: how many? Am J Med Genet. 2001;104(1):84 Epub 2001/12/18. . [DOI] [PubMed] [Google Scholar]
- 46.Farooq M, Ito M, Naito M, Shimomura Y. A case of monilethrix caused by novel compound heterozygous mutations in the desmoglein 4 (DSG4) gene. Br J Dermatol. 2011;165(2):425–31. Epub 2011 Jul 19. 10.1111/j.1365-2133.2011.10373.x [DOI] [PubMed] [Google Scholar]
- 47.Rakowska A, Rudnicka L. Monilethrix, Pseudomonilethrix, and Monilethrix-Like Hairs Atlas of Trichoscopy. London: Springer; 2012. p. 145–52. [Google Scholar]
- 48.Van Steensel M, Vreeburg M, Urbina MT, Lopez P, Morice-Picard F, Van Geel M. Novel KRT83 and KRT86 mutations associated with monilethrix. Exp Dermatol. 2015;24(3):222–4. 10.1111/exd.12624 [DOI] [PubMed] [Google Scholar]
- 49.Khan I, Maldonado E, Vasconcelos V, O’Brien SJ, Johnson WE, Antunes A. Mammalian keratin associated proteins (KRTAPs) subgenomes: disentangling hair diversity and adaptation to terrestrial and aquatic environments. BMC Genomics. 2014;15:779 Epub 2014/09/12. 10.1186/1471-2164-15-779 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farooq M, Ito M, Naito M, Shimomura Y. A case of monilethrix caused by novel compound heterozygous mutations in the desmoglein 4 (DSG4) gene. Br J Dermatol. 2011;165(2):425–31. Epub 2011/04/19. 10.1111/j.1365-2133.2011.10373.x . [DOI] [PubMed] [Google Scholar]
- 51.Balmer P, Bauer A, Pujar S, McGarvey KM, Welle M, Galichet A, et al. A curated catalog of canine and equine keratin genes. PLoS One. 2017;12(8):e0180359–e. 10.1371/journal.pone.0180359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19(24):6664–74. 10.1093/emboj/19.24.6664 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.FB UB, Cau L, Tafazzoli A, Mechin MC, Wolf S, Romano MT, et al. Mutations in Three Genes Encoding Proteins Involved in Hair Shaft Formation Cause Uncombable Hair Syndrome. Am J Hum Genet. 2016;99(6):1292–304. Epub 2016/11/22. 10.1016/j.ajhg.2016.10.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takizawa T, Takizawa T, Arai S, Kizawa K, Uchiwa H, Sasaki I, et al. Ultrastructural localization of S100A3, a cysteine-rich, calcium binding protein, in human scalp hair shafts revealed by rapid-freezing immunocytochemistry. J Histochem Cytochem. 1999;47(4):525–32. Epub 1999/03/20. 10.1177/002215549904700411 . [DOI] [PubMed] [Google Scholar]
- 55.Kizawa K, Troxler H, Kleinert P, Inoue T, Toyoda M, Morohashi M, et al. Characterization of the cysteine-rich calcium-binding S100A3 protein from human hair cuticles. Biochem Biophys Res Commun. 2002;299(5):857–62. Epub 2002/12/10. . [DOI] [PubMed] [Google Scholar]
- 56.Aoki N, Sawada S, Shimomura Y, Tsujimoto T, Ito K, Ito M, et al. A Novel Type II Cytokeratin, mK6irs, is Expressed in the Huxley and Henle Layers of the Mouse Inner Root Sheath. J Investig Dermatol. 2001;116(3):359–65. 10.1046/j.1523-1747.2001.01226.x [DOI] [PubMed] [Google Scholar]
- 57.Rogers MA, Winter H, Schweizer J, Langbein L, Praetzel S. K6irs1, K6irs2, K6irs3, and K6irs4 Represent the Inner-Root-Sheath-Specific Type II Epithelial Keratins of the Human Hair Follicle1. J Investig Dermatol. 2003;120(4):512–22. 10.1046/j.1523-1747.2003.12087.x [DOI] [PubMed] [Google Scholar]
- 58.Harel S, Christiano AM. Keratin 71 Mutations: From Water Dogs to Woolly Hair. J Investig Dermatol. 2012;132(10):2315–7. 10.1038/jid.2012.291 [DOI] [PubMed] [Google Scholar]
- 59.Bauer A, Hadji Rasouliha S, Brunner MT, Jagannathan V, Bucher I, Bannoehr J, et al. A second KRT71 allele in curly coated dogs. Anim Genet. 2018. 10.1111/age.12743 [DOI] [PubMed] [Google Scholar]
- 60.Cadieu E, Neff MW, Quignon P, Walsh K, Chase K, Parker HG, et al. Coat variation in the domestic dog is governed by variants in three genes. Science. 2009;326(5949):150–3. Epub 2009/08/29. 10.1126/science.1177808 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuramoto T, Hirano R, Kuwamura M, Serikawa T. Identification of the Rat Rex Mutation as a 7-bp Deletion at Splicing Acceptor Site of the Krt71 Gene. J Vet Med Sci. 2010;72(7):909–12. 10.1292/jvms.09-0554 [DOI] [PubMed] [Google Scholar]
- 62.Peters T, Sedlmeier R, Büssow H, Runkel F, Lüers GH, Korthaus D, et al. Alopecia in a Novel Mouse Model RCO3 Is Caused by mK6irs1 Deficiency. J Investig Dermatol. 2003;121(4):674–80. 10.1046/j.1523-1747.2003.12491.x [DOI] [PubMed] [Google Scholar]
- 63.Gandolfi B, Outerbridge CA, Beresford LG, Myers JA, Pimentel M, Alhaddad H, et al. The naked truth: Sphynx and Devon Rex cat breed mutations in KRT71. Mamm Genome. 2010;21(9–10):509–15. Epub 2010/10/19. 10.1007/s00335-010-9290-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujimoto A, Farooq M, Fujikawa H, Inoue A, Ohyama M, Ehama R, et al. A Missense Mutation within the Helix Initiation Motif of the Keratin K71 Gene Underlies Autosomal Dominant Woolly Hair/Hypotrichosis. J Investig Dermatol. 2012;132(10):2342–9. 10.1038/jid.2012.154 [DOI] [PubMed] [Google Scholar]
- 65.Cai J, Lee J, Kopan R, Ma L. Genetic interplays between Msx2 and Foxn1 are required for Notch1 expression and hair shaft differentiation. Dev Biol. 2009;326(2):420–30. Epub 2008/12/07. 10.1016/j.ydbio.2008.11.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johns SA, Soullier S, Rashbass P, Cunliffe VT. Foxn1 is required for tissue assembly and desmosomal cadherin expression in the hair shaft. Dev Dyn. 2005;232(4):1062–8. 10.1002/dvdy.20278 [DOI] [PubMed] [Google Scholar]
- 67.Johns SA, Soullier S, Rashbass P, Cunliffe VT. Foxn1 is required for tissue assembly and desmosomal cadherin expression in the hair shaft. Dev Dyn. 2005;232(4):1062–8. Epub 28 February 2005. 10.1002/dvdy.20278 [DOI] [PubMed] [Google Scholar]
- 68.Levy-Nissenbaum E, Betz RC, Frydman M, Simon M, Lahat H, Bakhan T, et al. Hypotrichosis simplex of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat Genet. 2003;34:151 https://www.nature.com/articles/ng1163#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 69.Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1:208 10.1038/35043032 [DOI] [PubMed] [Google Scholar]
- 70.Schlake T. FGF signals specifically regulate the structure of hair shaft medulla via IGF-binding protein 5. Development. 2005;132(13):2981–90. 10.1242/dev.01873 [DOI] [PubMed] [Google Scholar]
- 71.Schlake T. Segmental Igfbp5 expression is specifically associated with the bent structure of zigzag hairs. Mech Dev. 2005;122(9):988–97. 10.1016/j.mod.2005.04.012 [DOI] [PubMed] [Google Scholar]
- 72.Kljuic A, Bazzi H, Sundberg JP, Martinez-Mir A, O’Shaughnessy R, Mahoney MG, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113(2):249–60. Epub 2003/04/23. . [DOI] [PubMed] [Google Scholar]
- 73.Bazzi H, Demehri S, Potter CS, Barber AG, Awgulewitsch A, Kopan R, et al. Desmoglein 4 is regulated by transcription factors implicated in hair shaft differentiation. Differentiation. 2009;78(5):292–300. Epub 2009/08/18. 10.1016/j.diff.2009.06.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamakoshi T, Makino T, Ur Rehman M, Yoshihisa Y, Sugimori M, Shimizu T. Trichohyalin-like 1 protein, a member of fused S100 proteins, is expressed in normal and pathologic human skin. Biochem Biophys Res Commun. 2013;432(1):66–72. Epub 2013/02/05. 10.1016/j.bbrc.2013.01.084 . [DOI] [PubMed] [Google Scholar]
- 75.Adhikari K, Fontanil T, Cal S, Mendoza-Revilla J, Fuentes-Guajardo M, Chacón-Duque J-C, et al. A genome-wide association scan in admixed Latin Americans identifies loci influencing facial and scalp hair features. Nat Commun. 2016;7(10815). 10.1038/ncomms10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma L, Liu J, Wu T, Plikus M, Jiang T-X, Bi Q, et al. ‘Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003;130(2):379–89. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Godwin AR, Capecchi MR. Hoxc13 mutant mice lack external hair. Genes Dev. 1998;12(1):11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin ZCQ, Shi L, Giehl KA, Tang Z, Wang H, Zhang J, Yin J, Wu L, Xiao R, Liu X, Dai L, Zhu X, Li R, Betz RC, Zhang X, Yang Y. Loss-of-function mutations in HOXC13 cause pure hair and nail ectodermal dysplasia. Am J Hum Genet. 2012;91:906–11. 10.1016/j.ajhg.2012.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tkatchenko AV, Visconti RP, Shang L, Papenbrock T, Pruett ND, Ito T, et al. Overexpression of Hoxc13 in differentiating keratinocytes results in downregulation of a novel hair keratin gene cluster and alopecia. Development. 2001;128(9):1547–58. [DOI] [PubMed] [Google Scholar]
- 80.Abitbol M, Bosse P, Thomas A, Tiret L. A deletion in FOXN1 is associated with a syndrome characterized by congenital hypotrichosis and short life expectancy in Birman cats. PLoS One. 2015;10(3):e0120668 Epub 2015/03/18. 10.1371/journal.pone.0120668 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hwang J, Mehrani T, Millar SE, Morasso MI. Dlx3 is a crucial regulator of hair follicle differentiation and cycling. Development. 2008;135(18):3149–59. 10.1242/dev.022202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15(13):1688–705. 10.1101/gad.891401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guha U, Mecklenburg L, Cowin P, Kan L, O’Guin WM, D’Vizio D, et al. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165(3):729–40. 10.1016/S0002-9440(10)63336-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
BTS, bald thigh syndrome; C, control; m, male; mc, male castrated; f, female; fc, female castrated; SEM, scanning electron microscopy; WGS, whole genome sequencing.
(XLSX)
* Hairs were plucked from the hypotrichotic area on the border alopecia/haired.
(XLSX)
ID, gene identifier of the Entrez Gene database of NCBI; BaseMean, mean of normalized read counts across all samples; LfcSE, standard error of the log2 Fold Change; Stat, the log2 fold change divided by lfcSE; FDR, false discovery rate, refers to Benjamini-Hochberg adjusted p- value.
(XLSX)
ID, gene identifier of the Entrez Gene database of NCBI; Log2FC, log2 fold change; FDR, False discovery rate, refers to Benjamini-Hochberg adjusted p- value. Keratin expression in humans and sheep as reviewed in Harland, D.P. and Plowman, J.E. [3].
(XLSX)
ID, gene identifier of the Entrez Gene database of NCBI.; Log2FC, log2 fold change; FDR, false discovery rate, refers to Benjamini-Hochberg adjusted p- value.
(XLSX)
ID, gene identifier of the Entrez Gene database of NCBI; Log2FC, log2 fold change; FDR, false discovery rate, refers to Benjamini-Hochberg adjusted p- value; IRS, inner root sheath; HF, hair follicle; ORS, outer root sheath; DP, dermal papilla. [50, 52–55, 65, 66, 72–83].
(XLSX)
iBAQ, intensity based absolute quantification; LFQ, label free quantitation; top3, median normalized intensities of the three most intense peptides.
(XLSX)
Missing protein intensities were replaced sample wise by imputing a random number from the low end of the log2-transformed LFQ distribution; test differences were calculated on the median LFQ intensities (log2 transformed) and p values (-log) were corrected for multiple testing by a permutation based approach to estimate a 5% false discovery rate (q values); significant test results are marked by “+” in the “significant C vs BTS” column, if a protein in the control group is significantly higher expressed than in BTS and gene names of significantly differentially expressed proteins are highlighted in bold characters.
(XLSX)
0 stands for the wild type allele, 1 for the variant allele. Dog GY432 was excluded due to unknown phenotype.
(XLSX)
0 stands for the wild type allele, 1 for the variant allele. Dog GY432 was excluded due to unknown phenotype.
(XLSX)
0 stands for the wild type allele, 1 for the variant allele.
(XLSX)
(XLSX)
Samples are plotted across the two most variable components (PC1 and PC2) and sample clustering is based on condition.
(TIF)
Data Availability Statement
Data are available from the European Nucleotide Archive and GenBank (accession no. PRJEB21761 and PRJEB16012) as well as from ProteomeXchange via the PRIDE database (accession no. PXD012371). All other data are available in the paper and its Supporting Information files.