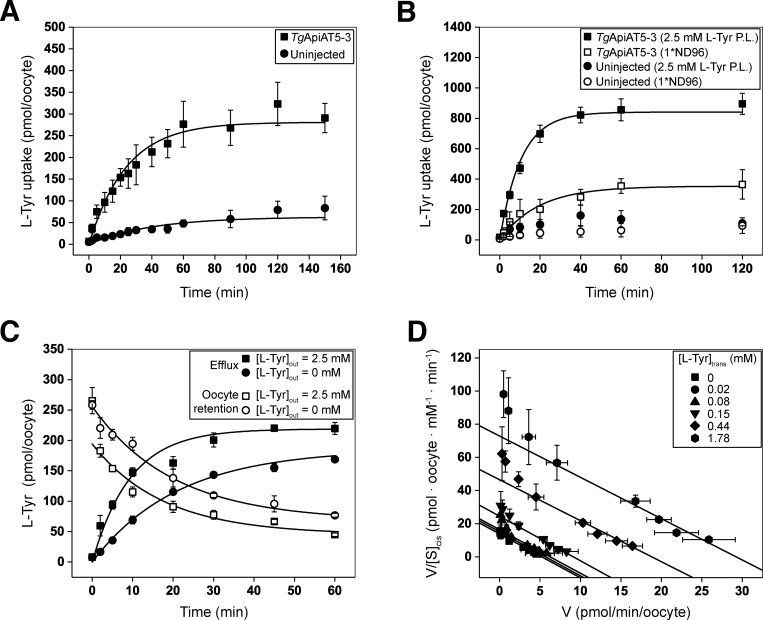
Fig 5. TgApiAT5-3 is an L-tyrosine transporter that is stimulated by the presence of L-tyrosine on the trans side of the membrane.
(A) Timecourse for the uptake of L-Tyr into X. laevis oocytes expressing TgApiAT5-3 (squares) or into uninjected oocytes (circles). Uptake was measured in the presence of 1 mM L-Tyr containing 0.5 μCi/ml [14C]Tyr. Each data point represents the mean uptake in 10 oocytes from a single experiment ± standard deviation, and the data are representative of 3 independent experiments. A first order rate equation was fitted to each timecourse (R2 = 0.97 for TgApiAT5-3-expressing oocytes and R2 = 0.77 for uninjected controls). Both the rate constant for L-Tyr uptake and the maximal L-Tyr uptake measured in TgApiAT5-3-expressing oocytes were significantly higher than those measured in uninjected oocytes (P < 0.01, Student’s t tests). (B) TgApiAT5-3-expressing oocytes (squares) and uninjected oocytes (circles) were preloaded (P.L.) with L-Tyr by incubation in 2.5 mM unlabelled L-Tyr (filled symbols) for 32 or 72 hr, respectively, or not preloaded (open symbols). Following the preincubation period, uptake of L-Tyr was measured in a solution of 1 mM L-Tyr containing 0.5 μCi/ml [14C]Tyr. Data show the mean uptake in 10 oocytes from a single experiment ± standard deviation, and are representative of 3 independent experiments. First order rate equations were fitted to the uptake timecourses for the preloaded and non-preloaded TgApiAT5-3-injected oocytes (R2 = 0.98 for preloaded, and R2 = 0.95 for non-preloaded oocytes). Both the first order rate constants for L-Tyr uptake and the maximal L-Tyr uptake were significantly higher in preloaded compared to non-preloaded TgApiAT5-3-expressing oocytes (P < 0.01, Student’s t tests). (C) TgApiAT5-3-expressing oocytes were preloaded by incubation in 1 mM L-Tyr containing 0.5 μCi/ml [14C]Tyr for 32 hr. Subsequent efflux (filled symbols) and retention (open symbols) of the preloaded substrate was measured over the timecourse indicated, in the presence of an extracellular medium containing 2.5 mM L-Tyr (squares) or extracellular medium lacking of L-Tyr (circles). Data show the mean efflux and retention ± standard deviation in 3 replicates (measuring efflux/retention from 5 oocytes each) from a single experiment, and are representative of 3 independent experiments. (D) Trans-stimulated initial rate kinetic analysis of L-Tyr transport by TgApiAT5-3. The rate of L-Tyr uptake was measured at a range of [L-Tyr] concentrations in the external medium (i.e. [L-Tyr]cis) in TgApiAT5-3-expressing oocytes preloaded with 0 mM to 2.5 mM L-Tyr (i.e. [L-Tyr]trans). The TgApiAT5-3-mediated uptake (calculated by subtracting the uptake in uninjected oocytes from the uptake in TgApiAT5-3-expressing oocytes) at each [L-Tyr]trans condition tested conformed to a Michaelis-Menten kinetic model (R2 > 0.90 for all non-linear regressions). The data were fitted to a Scatchard linear regression (0.89 ≤ R2 ≤ 0.98 for all linear regressions). Data show the mean uptake rate ± standard deviation in 10 oocytes from a single experiment, and are representative of 2 independent experiments.