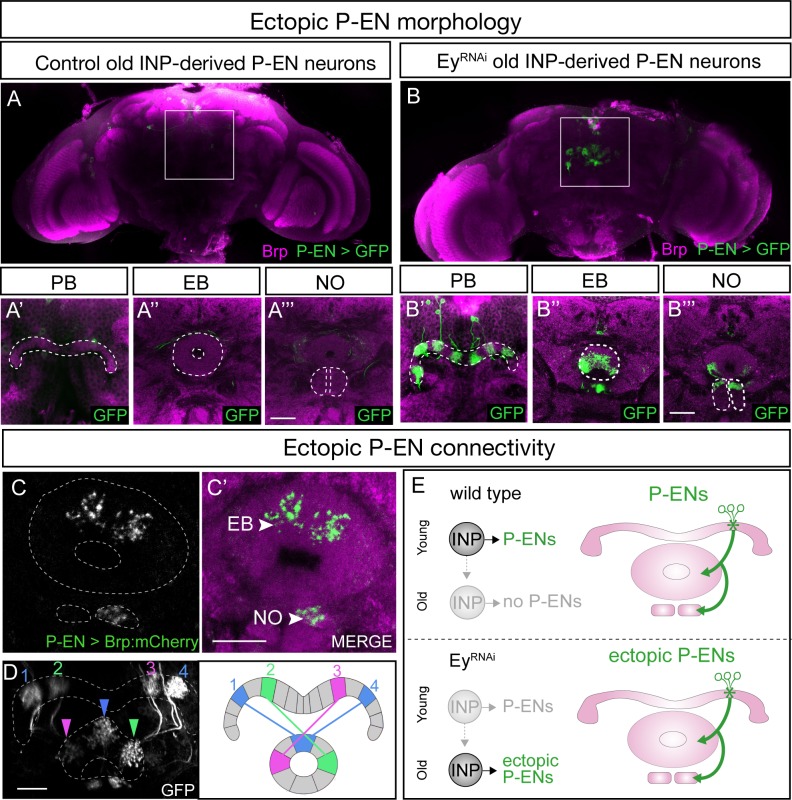
Figure 6. EyelessRNAi produces late-born ‘ectopic’ P-EN neurons that have normal P-EN morphology and connectivity.
(A–A’’’) In wild-type adults, late INP clones do not label P-EN neurons in the adult brain (n = 5). See Materials and methods for details. PB, EB, and NO neuropils marked with dashed lines. Scale bars, 20µm (A–B). (B–B’’’) In EyelessRNAi adults, late INP clones produce ectopic late-born P-EN neurons, which project to the PB, EB, and Noduli (n = 5), similar to endogenous P-EN neurons (Figure 1A,B). PB, EB, and NO neuropils marked with dashed lines. (C–C’) In EyelessRNAi adults, late INP clones produce ectopic late-born P-EN neurons, which localize the pre-synaptic marker Brp::mCherry to the EB and Noduli (n = 5), but not to the PB (not shown), similar to the endogenous P-EN neurons. Scale bars, 20µm (C–D). (D) EyelessRNAi adult, showing stochastic labeling of four ectopic P-EN neurons (1-4) with normal PB and EB glomeruli targeting (compare to Wolff and Rubin, 2018). (E) Summary.
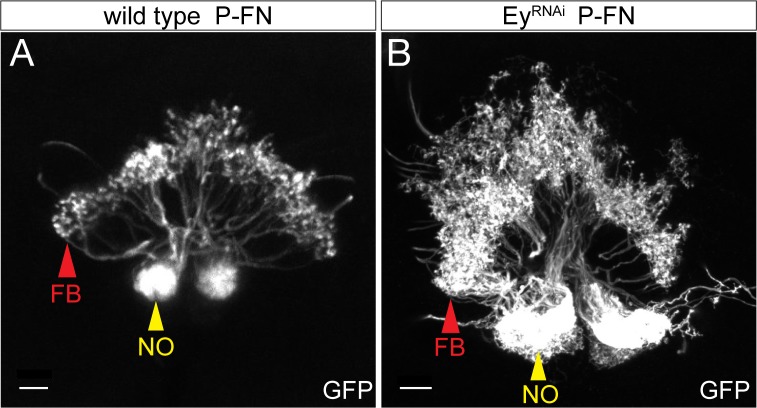
Figure 6—figure supplement 1. EyelessRNAi produces late-born ‘ectopic’ P-FN neurons that have normal P-FN morphology.
Figure 6—figure supplement 2. Eyeless regulation of identity schematic.