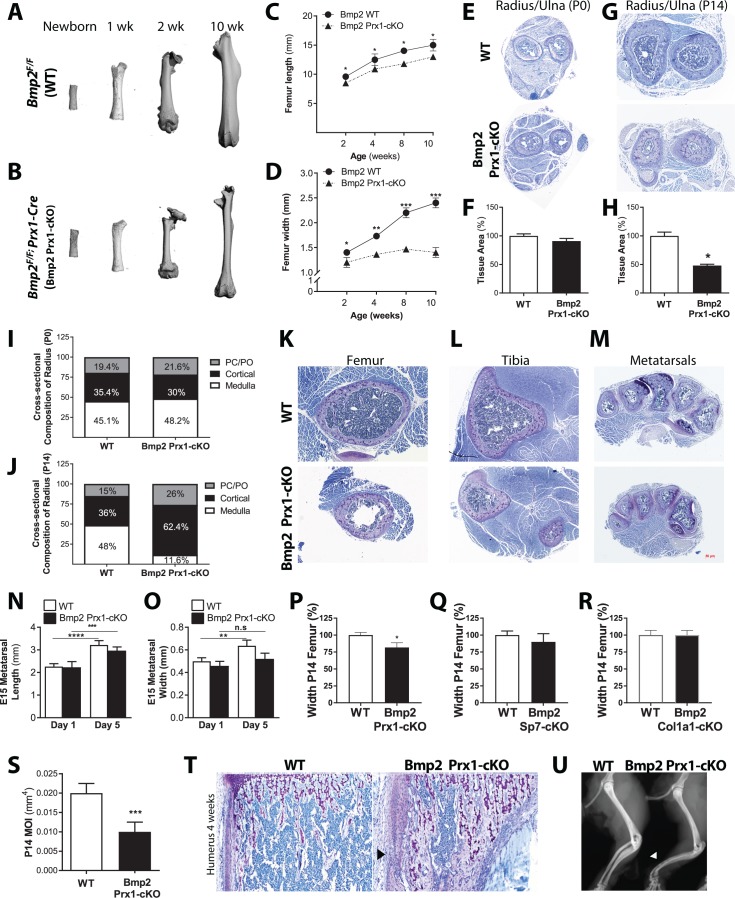
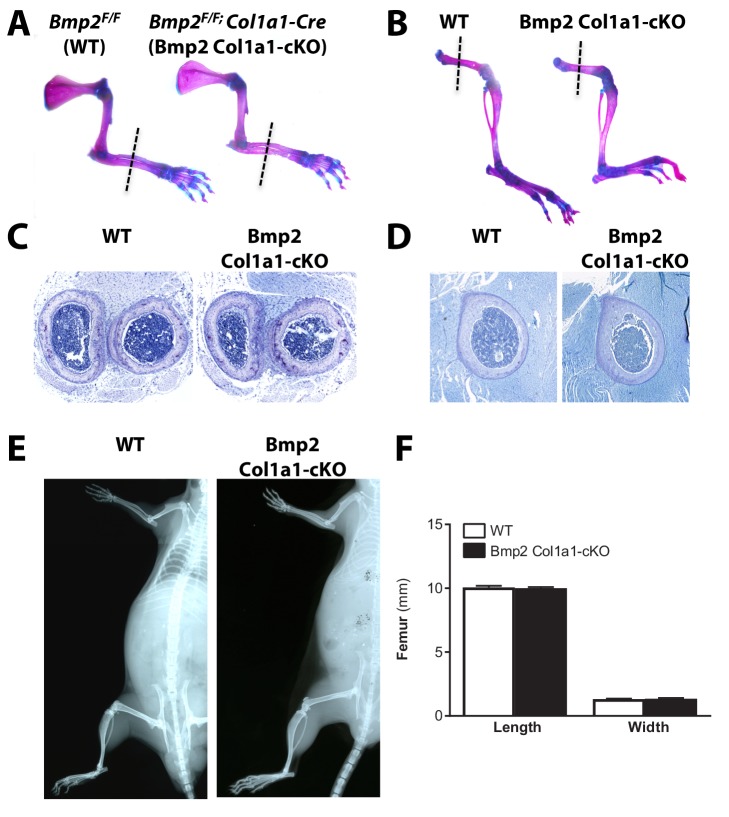
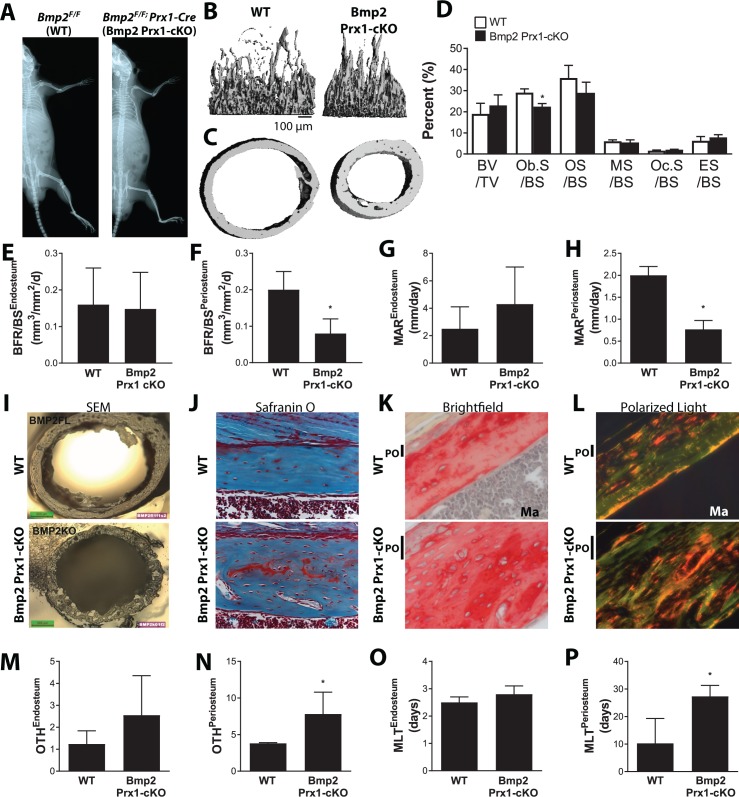
Figure 1. Osteoprogenitor-derived Bmp2 couples length to width in the appendicular skeleton.
(a,b) Representative 3D reconstructions of the murine femur using microcomputed tomography (microCT). (c) Femoral length or (d) femoral width at mid-diaphysis, presented as mean ± s.d. with n = 8–20 bones per age per genotype. *p<0.05, **p<0.005, or ***p<0.0005 vs. age-matched Bmp2 Prx1-cKO cohort. (e,g) Representative toluidine blue histology at the mid-diaphysis of the forelimb. (f,h) MicroCT analysis of total cross-sectional bone tissue area presented as mean ±s.d. with n = 4. *p>0.05. (i,j) Cross-sectional composition of cortical bone in the radius of newborn (n = 6–9 per genotype) or 2 week-old mice (n = 3–9 per genotype) (see Materials and methods). Abbreviations: PC, perichondrium; PO, periosteum. (k,l,m) Representative toluidine blue histology at the mid-diaphysis of indicated skeletal elements. (n) Length or (o) width of embryonic day 15 metatarsals, measured following 1 or 5 days of ex vivo culture. Mean ±s.d. with n = 6–12 where **p<0.005, ***p<0.005, or ****p<0.00005. (p,q,r) Femur width mean ±s.d. with n = 6–12 where *p<0.05. (s) Minimum moment of inertia in P14 femur, calculated by microCT (n = 4) shown as mean ±s.d. where ***p<0.0005. (t) Toludine blue histology revealing cortical microcracks in the humerus of Bmp2 Prx1-cKO mice at 4 weeks of age. (u) X-ray images showing representative bowing of the radius and ulna of Bmp2 Prx1-cKO mice in the absence of frank fractures. Statistical analyses were performed using two-tailed Student’s t-test.