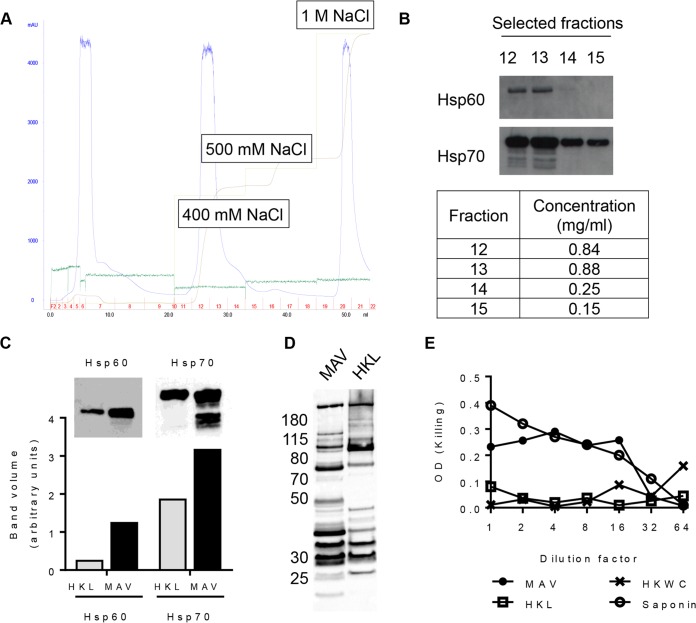
FIG 1.
Formulation of a multiple-antigen S. pneumoniae TIGR4-derived vaccine preparation (MAV). (A) Ion-exchange (IEX) chromatogram showing the purification of the MAV. Light green line, NaCl elution concentration; brown line, the resulting conductivity in the system; blue line, UV trace showing the concentrations of the eluted proteins (milli-absorbance units); dark green line, pressure in the system. The fractions collected are numbered in red; the total volume (in milliliters) is recorded on the x axis. (B) Detection of Hsp60 and Hsp70 by Western blotting in selected ion-exchange chromatography fractions; the BCA assay protein concentrations for these fractions are shown in the table. (C) A comparison of the heat shock protein content (Hsp60 and Hsp70) measured by immunoblotting of heat-killed lysate (HKL) and MAV. The bar chart shows the pixel intensity quantification (ImageQuant TL; GE Lifesciences) for Hsp60 and Hsp70 bands. (D) Immunoblots of 5 μg of total protein of either MAV or HKL probed with pooled human IgG at 1:20,000 (Pentaglobin; Paviour Pharmaceuticals, New Delhi, India). (E) Comparison of the hemolytic activity against horse red blood cells in serial 2-fold dilutions of MAV from neat to 1:64 and HKL and HKWC preparations with a saponin positive control.