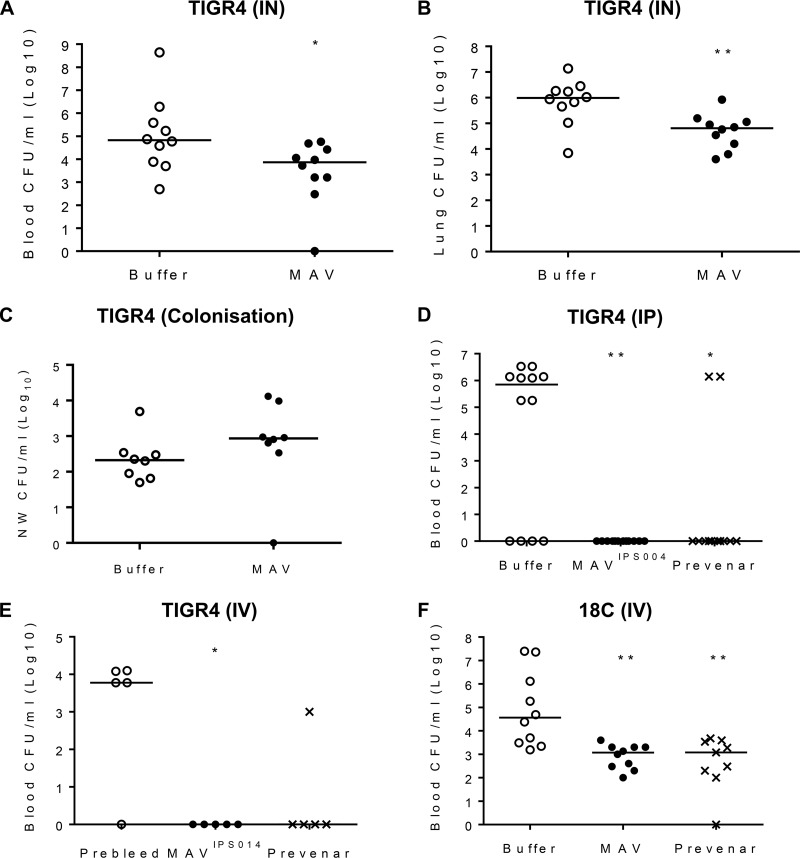
FIG 6.
Vaccination with MAV preparations protects mice against S. pneumoniae challenge. (A and B) Number of CFU in the lung (A) and blood (B) 24 h after challenge by intranasal (IN) inoculation with 1 × 107 CFU of the S. pneumoniae TIGR4 strain in mice vaccinated twice subcutaneously with 75 μg of MAV or a negative-control buffer (n = 10 per group). (C) Number of CFU in nasal wash (NW) specimens 2 weeks after nasopharyngeal colonization with 5 × 106 CFU of S. pneumoniae TIGR4 of mice vaccinated twice subcutaneously with 75 μg of MAV or a negative control buffer (n = 8 per group). (D) Number of CFU in blood 6 h after challenge by intraperitoneal (IP) inoculation of 1 × 104 CFU of the S. pneumoniae TIGR4 strain into mice passively vaccinated with 200 μl of sera from rabbits vaccinated three times with 375 μg of MAVIPS004 or twice with 0.2 ml of the Prevenar vaccine or a negative-control buffer (n = 12 per group). (E and F) Number of CFU in the blood of mice 4 h after challenge by intravenous (IV) inoculation with 5 × 105 CFU of the S. pneumoniae TIGR4 (E) or ATCC BAA-1662 (18C) (F) strain that was incubated preinoculation in sera obtained from rabbits vaccinated with MAVIPS014, the Prevenar vaccine, or a negative-control buffer (n = 5 to 10). For all panels, each symbol represents data from a single mouse, and horizontal bars represent median values. Statistical significances were calculated using a Mann-Whitney t test (A to D) or Dunnett’s multiple-comparison test (E and F). Significance abbreviations: *, P < 0.05; **, P < 0.01.