Figure 7.
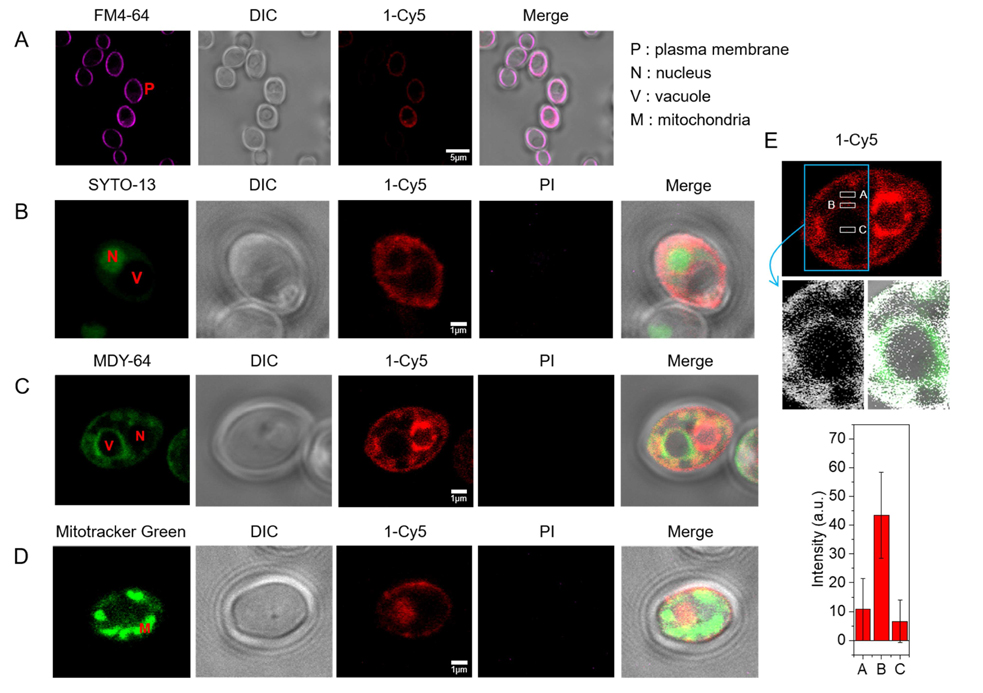
Subcellular localization of β-peptide 1-Cy5. 2 µM β-peptide 1-Cy5, 3 µM unlabeled β-peptide 1 and an organelle specific dye (1 µg/mL FM 4–64 for plasma membrane, 2.5 µM SYTO 13 for nucleus, 0.5 µM Mitotracker Green for mitochondria, and 5 µM MDY 64 for intracellular membranes, and 1 µg/mL PI for dead cell) were incubated with C. albicans cells for 5 – 30 min, followed by β-peptide incubation, and then imaged. (A) C. albicans cells were incubated with FM 4–64 for 5 min (34 of 34 cells in three experiments exhibited plasma membrane localization of β-peptide). (B) Cells were incubated with SYTO 13 for 20 min (10 of 21 cells in three experiments exhibited nuclear localization). (C) Cells were incubated with MDY 64 for 5 min (11 of 25 cells in three experiments exhibited vacuolar localization). (D) Cells were incubated with Mitotracker green for 30 min (0 of 50 cells in three experiments exhibited mitochondrial localization). (E) Quantitation of 1-Cy5 fluorescence in three areas near the vacuole: A (cytosol), B (vacuole membrane), and C (center of vacuole). Top image shows the whole cell with regions used for fluorescence quantitation outlined in white. Blue indicates the cropped vacuole region (below, white represents 1-Cy5, and green represents MDY-64). The plot in the bottom of the panel shows average fluorescence intensities in regions A, B, and C taken from measurements on images of 11 different cells similar to the representative images shown here. Scale bars indicates 5 µm for (A) and 1 µm for (B), (C), and (D). P labels the plasma membrane, N the nucleus, V the vacuole, and M the mitochondria. See also Movie S1.
