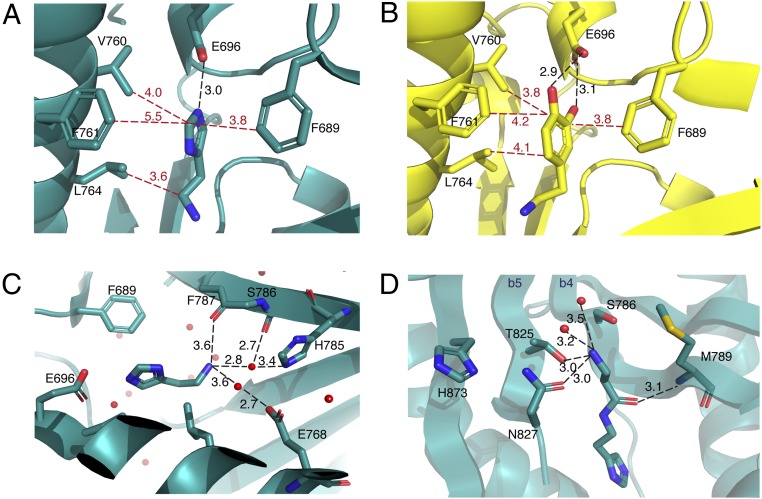
Fig. 3.
Structures of substrate- and product-bound Ebony CN and residues involved in orientation of the substrates. (A and B) Cartoon representation of histamine-bound (A) and dopamine-bound (B) Ebony CN, with residues forming the “hydrophobic cage.” Hydrophobic interactions are in red; hydrogen-bonding interactions, in black. (C) Cartoon representation of residues playing a role in positioning the amine substrate (histamine-bound CN structure shown). These include the backbone carbonyl groups of F787 and S786 together with the side chains of residues H785 and E768 (T825 omitted for clarity). (D) Cartoon representation of the hydrophilic substrate-binding channel (carcinine-bound CN structure shown), indicating how hydrogen-bonding interactions stabilize the amine in the β-alanine moiety of carcinine. The proximity of the N827 side chain relative to carcinine explains why compound 6 is not accepted as a substrate but its enantiomer (7) is (due to the cavity between carcinine and the M789 side chain accommodating the methyl branch of 7).