Fig. 7.
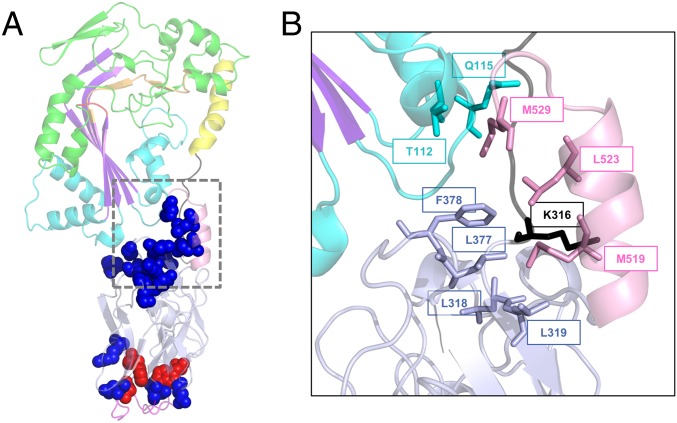
Functionally important positions within the C-terminal domain of GNIP1Aa. (A) Ribbon representation of the GNIP1Aa protein. The same colors were used as in Fig. 1A, displayed at 50% transparency. All positions shown in sphere representation are sensitive to a single substitution with an alanine: replacement of residues in red leads to complete knockout of protein WCR toxicity, and in blue to the reduced protein activity (total of 17). See Table 1 and SI Appendix, Fig. S3 for details. (B) Close-up of the extensive interaction of the selected C-terminal residues with different protein modules, shown in ribbon representation. The M519, L523, and M529 of the C-terminal helix are in contact with four protein regions. The aforementioned residues and their interacting partners are shown in stick representation, labeled with a corresponding position number, and colored the same as in Fig. 1A.