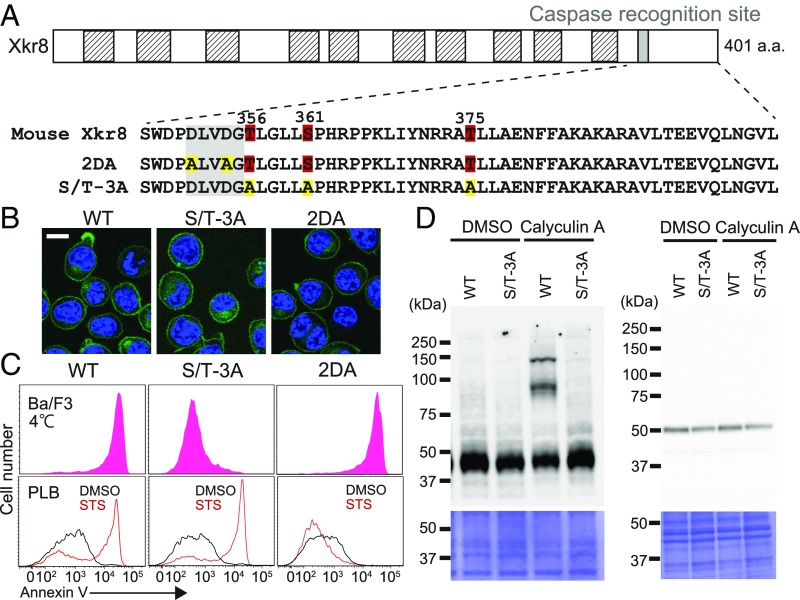
Fig. 3.
Assignment of the phosphorylation sites in mXkr8. (A) Phosphorylation sites in mXkr8. The structure of mXkr8 is shown schematically. Putative transmembrane regions are shown in hatched boxes, and the caspase 3 recognition motif is shown as a filled box. The amino acid sequence of the caspase recognition motif and its downstream region is shown. The phosphorylated residues (Thr-356, Ser-361, and Thr-375) are numbered. In the 2DA mutant, Asp-351 and Asp-354 in the caspase recognition motif were mutated to Ala, and in the S/T-3A mutant, Thr-356, Ser-361, and Thr-375 were mutated to Ala. (B) Localization of Xkr8 mutants to the plasma membrane. The WT and indicated mutants of mXkr8 were fused to EGFP and expressed in TMEM16F−/−Xkr8−/−BaF (DKO-BaF) cells. The stable transformants were stained with 5.0 μg/mL Hoechst 33342 and observed by confocal microscopy for GFP (green) and Hoechst 33342 (blue). (Scale bar: 10 μm.) (C) Kinase-dependent but caspase-independent PtdSer exposure by mXkr8. (Upper) DKO-BaF cells expressing WT, S/T-3A, or 2DA Xkr8 were stained at 4 °C for 15 min with Cy5-annexin V. The annexin V-staining profiles in the GFP-positive and PI-negative population are shown. (Lower) Human PLB cells expressing WT, S/T-3A or 2DA were treated at 37 °C for 120 min with 10 μM STS and then stained with annexin V at 4 °C. (D) Effect of the S/T-3A mutation on the phosphorylation of mXkr8. (Upper) DKO-BaF transformants (1 × 106 cells) expressing WT or S/T-3A mutant mXkr8 were incubated at 37 °C for 30 min with or without 50 nM calyculin A. After solubilization of the cells with RIPA buffer, aliquots of the lysates corresponding to 4 × 104 cells were separated by 8% Phos-tag PAGE (Left) or 10–20% SDS/PAGE (Right) and then analyzed by Western blot analysis with anti-GFP. (Lower) The membranes were stained with Coomassie brilliant blue.