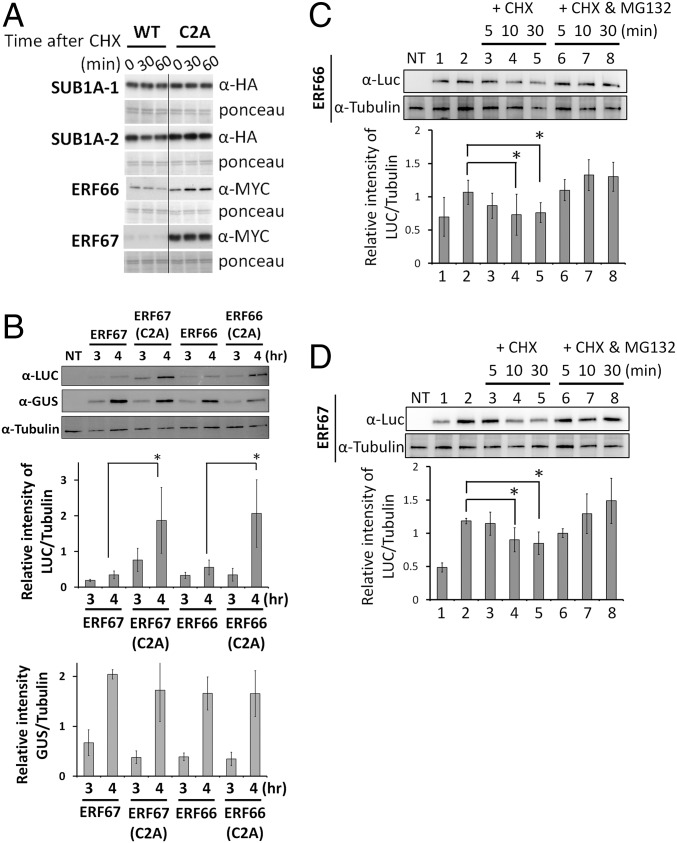
Fig. 4.
ERF66 and ERF67 are substrates of the N-end rule pathway. (A) In vitro analysis of protein stability of HA- or MYC-tagged WT and C2A variants of SUB1A-1, SUB1A-2, ERF66, and ERF67 following treatment with CHX. (B) The stability of ERF66 and ERF67 expressed in rice protoplasts is enhanced by a C2A mutation or treatment with MG132. UbiP::ERFVII-Luc constructs were cotransfected into TNG67 rice protoplasts with a UbiP::GUS plasmid, which was used as a stable control. The transfected protoplasts were incubated in W5 solution for 3 or 4 h and then harvested for Western blot analysis (SI Appendix, Fig. S7A), and the relative levels of Luc and GUS were normalized to tubulin, respectively. NT, nontransfected. The molecular weights of ERF66/67-Luc fusion proteins are ∼87/85 kDa, respectively. (C and D) CHX chase of ERF66 and ERF67 with/without MG132 in rice protoplasts. The protein levels of ERF66/ERF67 4 h after transformation without MG132 (lane 1) and with MG132 (lane 2) are shown. CHX chase experiments were initiated after 4 h treatment with MG132 to ensure high levels of protein at the beginning of the chase by replacing buffer with CHX only (100 μM) or CHX and MG132 (20 μM). The relative levels of Luc were normalized to tubulin. The data represent means ± SD from three independent replicates (*P < 0.05 indicates a significant difference by Student’s t test).