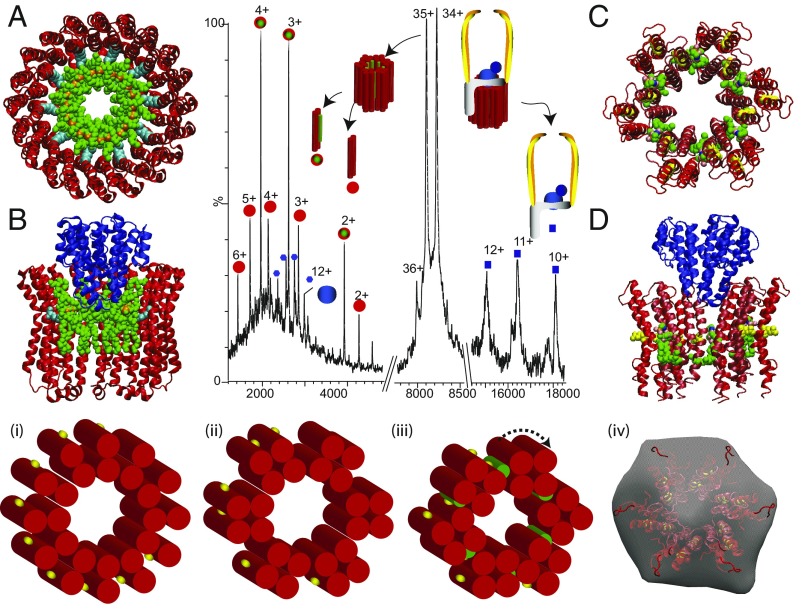
Fig. 2.
Tandem MS of a subcomplex from T. thermophilus ATPase (ICL12E2G2F) leads to disruption of the L12 ring, releasing proteolipids L ± PE (red/green circle, 8,539 Da; red circles, 7,849 Da) and a stripped-complex ICE2G2F (blue squares, 184,242 Da). Atomic structure of the K10 ring of E. hirae ATPase (50) with docking of 10 cardiolipins to show reduction in the inner diameter (A) and after docking subunit C (B). Models for sixfold symmetry of the L12 ring with six PE molecules (green) (C) and with subunit C (blue) (51) docked into the ring (D). (Lower) Schematics of the rotor ring with 12-L subunits each having two transmembrane helices (red cylinders) and one conserved glu-63 (yellow) as seen in electron microscopy of 2D crystals (52) (i). Transformation into a sixfold symmetric ring (ii and iii). (iv) Comparison of the sixfold symmetrical model with electron microscopy data reported previously for the T. thermophilus ATPase (iv) (53).