Fig. 3.
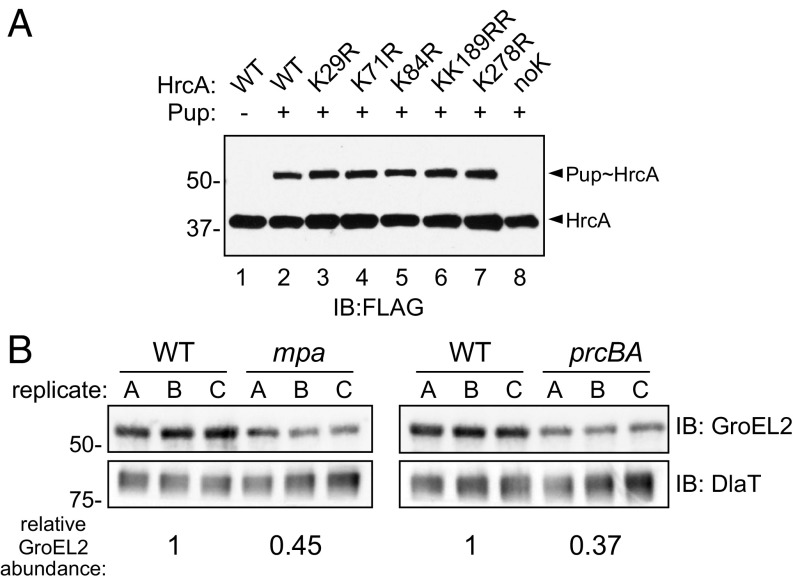
HrcA is a pupylated protein that is likely degraded by the M. tuberculosis proteasome. (A) Purified HrcA can be pupylated on any of its lysines by PafA. His6-PupGlu and PafA-His6 were coincubated with HrcATAP WT or lysine-to-arginine (K>R) variants, and both native and pupylated HrcA were detected by immunoblotting (IB) using an antibody that recognizes an affinity tag on HrcATAP (FLAG). Data are representative of three independent experiments. (B) GroEL2 abundance is low in both mpa (MHD149) and prcBA strains compared with the WT parental strain. Immunoblots for GroEL2 and dihydrolipoamide acyltransferase (DlaT) were performed on the same membrane using samples obtained from replicate PB-nitrate cultures. For each lane, GroEL2 was normalized to DlaT, a protein that is not regulated by the PPS. The difference in normalized GroEL2 abundance between strains is indicated at the Bottom; for comparison of WT and mpa strains, this difference had a P value of 0.07; the difference in GroEL2 abundance between WT and prcBA strains had a P value of <0.01.