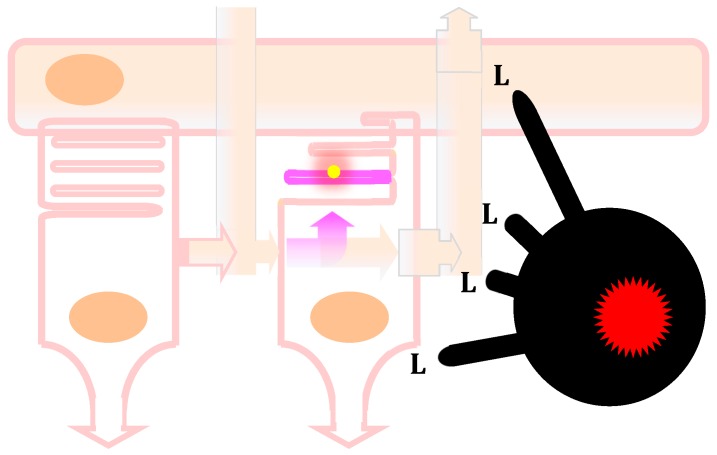
Figure 3.
Blood-derived inflammatory macrophage perturbation of the metabolic ecosystem between photoreceptors and the retinal pigmented epithelium. In non-pathological conditions, the rod on the left produces and secretes the truncated thioredoxin rod-derived cone viability factor (RdCVF, pink arrow toward the right). The cone on the right expresses the RdCVF cell-surface receptor basigin-1 (BSG1). RdCVF activates the complex formed between BSG1 and the glucose transporter GLUT1 (SLC2A1) resulting in an acceleration of the entry of glucose coming from the blood circulation through the retinal pigmented epithelium (RPE, on the top). The cone metabolizes glucose through aerobic glycolysis that produces glycerol-3-phosphate as a precursor of the hydrophilic head of the phospholipids (dark pink) for the renewal of the cone outer segment that contains the light-sensing molecule, the opsin (yellow). Aerobic glycolysis also produces lactate that is transported outside the cone by a lactate (lac) transporter. The lactate is partially transported through the RPE toward the blood circulation via two lactate transporters; the one on the basal side (on the top) is encoded by the SLC16A8 gene that carries risk alleles for AMD. A certain proportion of the transported lactate is metabolized by the RPE to pyruvate that fuels the mitochondrial oxidative phosphorylation. In that ecosystem, the glucose issued from the blood circulation is not metabolized by the RPE. In pathological conditions, as in patients carrying SLC16A8 risk alleles for AMD, the accumulation of lactate and its metabolism by the mitochondrial respiratory chain produces an excess of reactive oxygen species by leakage, and since lactate transporters are facilitating transporters, the rise in lactate in the RPE triggers an elevation of lactate in the extracellular space between photoreceptors. For the same mechanistic reason, an excess of lactate outside the cone counteracts the intracellular glycolytic flux and inhibits aerobic glycolysis, resulting in the shortening of the cone outer segment and the impairment of cone vision of the macula. This AMD pathological mechanism, identified through genome-wide association studies, may represent a genetic signature of a role of lactate produced after the metabolism reprogramming of inflammatory macrophages occurring in the disease due to chronic inflammation. This is illustrated by the infiltrated inflammatory macrophage (black, on the far right) which produces lactate and elevates the concentration of lactate in the retina, as does the SLC16A8 risk alleles.