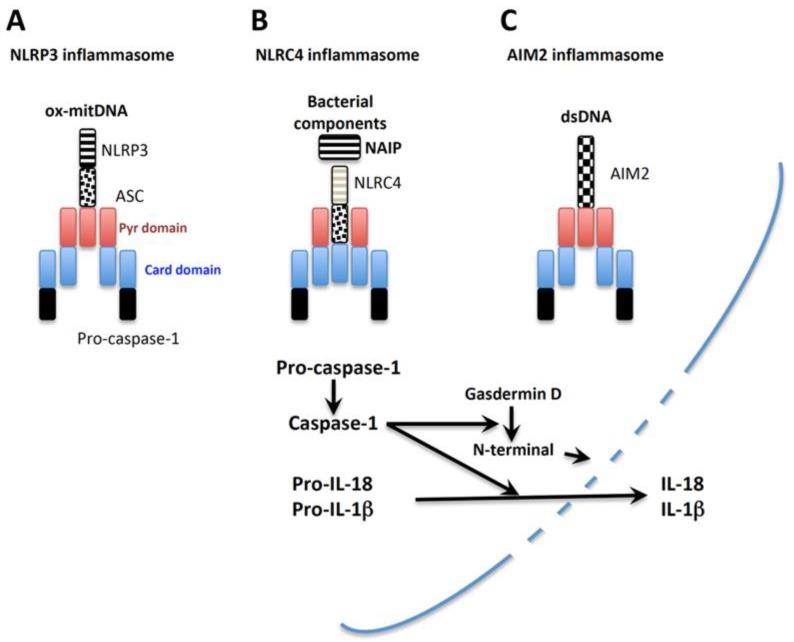
Figure 2.
Representative inflammasomes. Caspase-1, an IL-1β/IL-18-converting enzyme, is produced as an enzymatically inactive precursor (pro). Upon the appropriate stimulation, many cell types including macrophages and epithelial cells, form cytoplasmic machinery termed the inflammasome for the activation of pro-caspase-1. Several cytoplasmic pattern-recognition receptors including NLR and AIM2-like receptors, serve as a scaffold molecule for individual inflammasome activation. (A) The NLRP3 inflammasome. After recognition of oxidized mitochondrial (ox-mit) DNA, NLRP3, a member of the NLR, assembles a caspase activating adaptor, ASC, and pro-caspase-1, which eventually leads to active caspase-1. (B) The NLRC4 inflammasome. After recognition of corresponding bacterial secretion system III, components from human NAIP or murine NAIP family members associate with NLRC4, which results in formation of the NLRC4 inflammasome. (C) The AIM2 inflammasome. After the recognition of double-stranded (ds) DNA, AIM2 similarly generates the AIM2 inflammasome. Active caspase-1 then cleaves pro-IL-18 and pro-IL-1β into IL-18 and IL-1β, respectively. Caspase-1 also cleaves gasdermin D into a pore-forming N-terminal protein. IL-18 and IL-1β as well as other cytoplasmic proteins including HMGB1, are extracellularly released through membrane pores generated by the N-terminal protein. Blue line indicated the cell membrane.