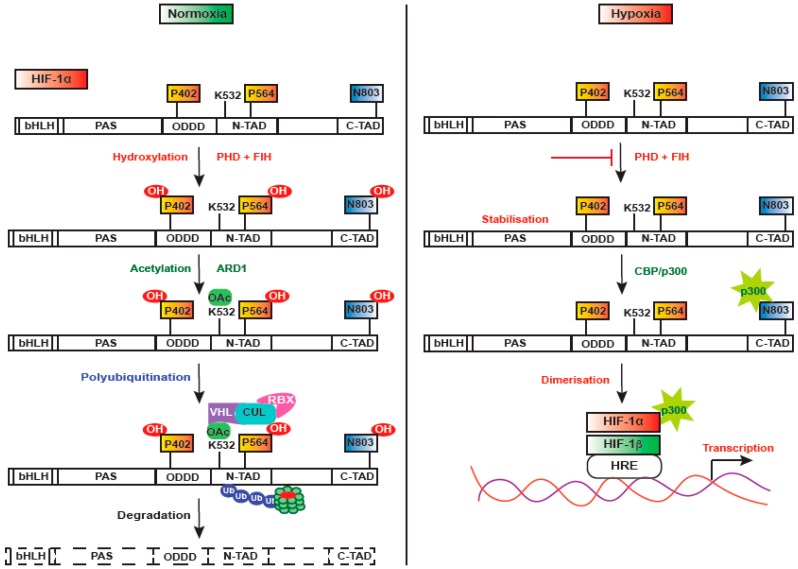
Figure 1.
Regulation of HIF-1alpha under normoxia and hypoxia. In normoxia, P402 and P564 proline residues and N803 residue are hydroxylated by prolyl hydroxylases (PHDs) and factor inhibiting HIF-1 (FIH-1), respectively, in an O2-dependent manner, followed by ADP-ribosylation factor domain protein-1 (ARD1) dependent Acetylation of K532 lysine residue. Hydroxylated HIF-1 then binds to VHL E3 ubiquitin ligase complex, leading to its proteasomal degradation. Hydroxylated N803 blocks the recruitment of the CBP/P300 transcriptional coactivator, whereas during hypoxia, PHDs and FIH-1 are blocked, thus inhibiting the hydroxylation of proline and asparagine residues. Lack of hydroxylation prevents the binding of VHL, thus stabilizing HIF-1alpha, which translocates into the nucleus, allowing the recruitment of CBP/P300 and gene transcription.