Figure 2.
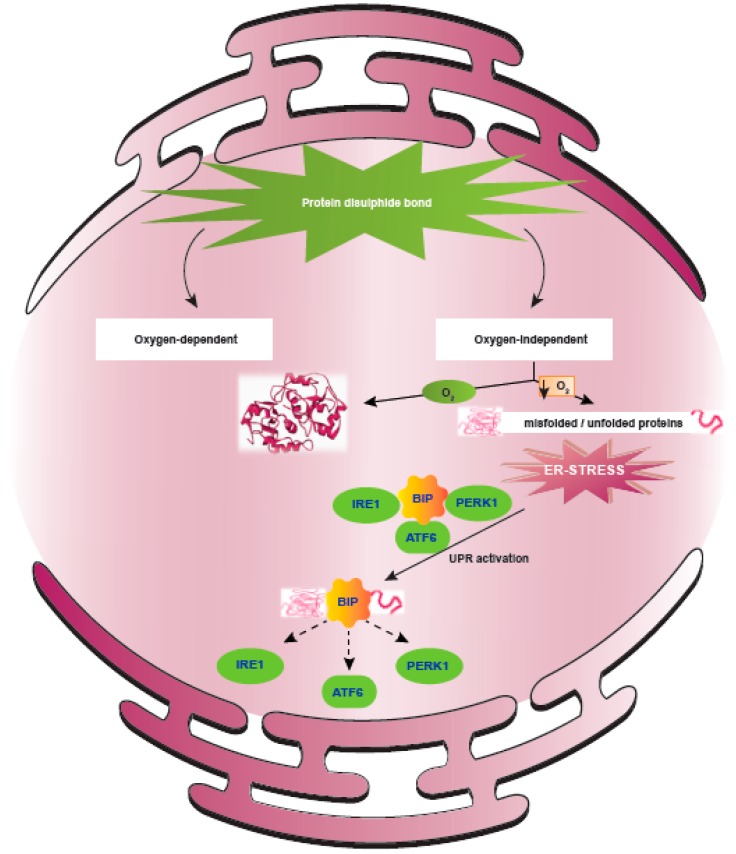
Hypoxia induced ER stress. Endoplasmic reticulum is the central organelle responsible for protein translational modifications, wherein the formation of protein disulphide bond is mediated by protein disulphide isomerase (PDI), an ER chaperone. During protein synthesis, protein disulphide bond is independent of oxygen, but protein folding is dependent on oxygen. Hence, in solid tumors, decreased availability of oxygen (hypoxic regions/fraction) causes perturbations in protein folding and results in the accumulation of misfolded/unfolded proteins. These changes disturb the ER proteostasis, leading to “ER Stress” and activation of unfolded protein response (UPR) as an adaptive mechanism.