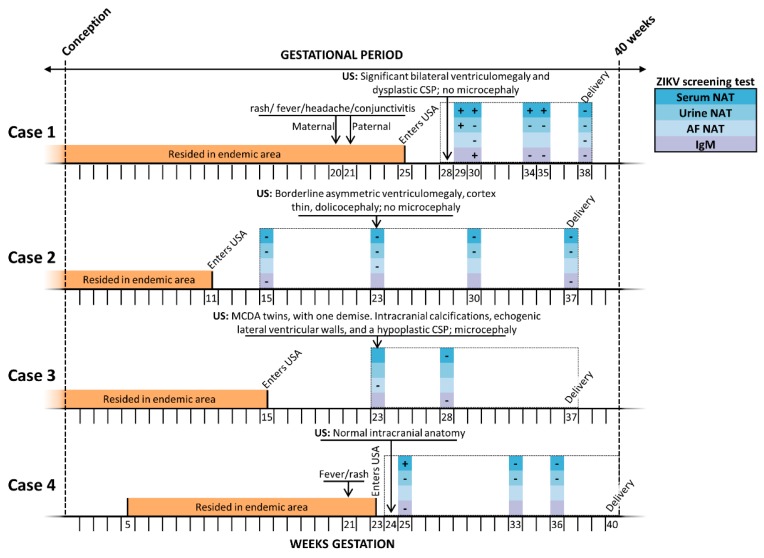
Figure 1.
Timeline of subjects’ exposure risk, clinical findings, and standard clinical laboratory testing for ZIKV. The entire gestational interval during which antenatal care was delivered is outlined by a checked box, with annotated possible windows of endemic exposure (orange bar) and post-emigration ZIKV maternal screening (nucleic acid testing (NAT) or serologic IgM), colored by type and fluid tested (blue/purple legend). Estimated date of delivery (EDD, 40 weeks’ gestation; dashed line far right) was calculated based on obstetrical estimate, and confirmed by clinical best practice sonographic measures; interval week of gestational age is denoted on the X axis. Key findings by prenatal ultrasound (US) and/or MRI and gestational age at delivery are shown. CSP—cavum septum pellucidum; MCDA—monochorionic, diamniotic twin gestation; NAT—nucleic acid testing.