Abstract
The plant Cannabis sativa L. has been used as an herbal remedy for centuries and is the most important source of phytocannabinoids. The endocannabinoid system (ECS) consists of receptors, endogenous ligands (endocannabinoids) and metabolizing enzymes, and plays an important role in different physiological and pathological processes. Phytocannabinoids and synthetic cannabinoids can interact with the components of ECS or other cellular pathways and thus affect the development/progression of diseases, including cancer. In cancer patients, cannabinoids have primarily been used as a part of palliative care to alleviate pain, relieve nausea and stimulate appetite. In addition, numerous cell culture and animal studies showed antitumor effects of cannabinoids in various cancer types. Here we reviewed the literature on anticancer effects of plant-derived and synthetic cannabinoids, to better understand their mechanisms of action and role in cancer treatment. We also reviewed the current legislative updates on the use of cannabinoids for medical and therapeutic purposes, primarily in the EU countries. In vitro and in vivo cancer models show that cannabinoids can effectively modulate tumor growth, however, the antitumor effects appear to be largely dependent on cancer type and drug dose/concentration. Understanding how cannabinoids are able to regulate essential cellular processes involved in tumorigenesis, such as progression through the cell cycle, cell proliferation and cell death, as well as the interactions between cannabinoids and the immune system, are crucial for improving existing and developing new therapeutic approaches for cancer patients. The national legislation of the EU Member States defines the legal boundaries of permissible use of cannabinoids for medical and therapeutic purposes, however, these legislative guidelines may not be aligned with the current scientific knowledge.
Keywords: Cannabinoids, antitumor effects, signaling pathways, legislation
INTRODUCTION
The first discovered and most important source of cannabinoids was the plant Cannabis sativa L., which has been used as an herbal remedy for centuries. The earliest archaeological evidence of cannabis medical use dates back to the Han Dynasty in ancient China, where it was recommended for rheumatic pain, constipation, disorders of the female reproductive tract, and malaria among other conditions. In traditional Indian Ayurvedic medicine, cannabis was used to treat neurological, respiratory, gastrointestinal, urogenital, and various infectious diseases [1]. The plant was also cultivated in other countries in Asia as well as in Europe, especially for making ropes, clothes/fibres, food and paper [2]. In Western medicine, the use of cannabis was notably introduced by the work of William B. O’Shaughnessy (an Irish physician) and Jacques-Joseph Moreau (a French psychiatrist) in the mid-19th century, who described positive effects of cannabis preparations, including hashish (the compressed stalked resin glands), on pain, vomiting, convulsions, rheumatism, tetanus and mental abilities. Cannabis was recognized as a medicine in the United States (US) Pharmacopoeia from 1851, in the form of tinctures, extracts and resins. However, in the beginning of the 20th century, cannabis use decreased in Western medicine due to several reasons: increased use as a recreational drug, abuse potential, variability in the quality of herbal material, individual (active) compounds were not identified and alternative medications, with known efficacy, were introduced to treat the same symptoms [2,3]. In 1941, as the result of many legal restrictions, cannabis was removed from the American Pharmacopoeia and considered to be in the same group as other illicit drugs [3]. Consequently, the exploration of medical uses of cannabis has been significantly slowed down for more than a half of century. In 2013, a step forward was made with the inclusion of a monograph of Cannabis spp. in the American Herbal Pharmacopoeia [4]. Moreover, the current legislative changes in the European Union (EU), US and Canada that allow cannabis for medical and/or recreational use, the progress in scientific research and public awareness on the benefits of medical cannabis all contributed to the rising interest in the therapeutic potential of cannabinoids [5,6].
In recent years, cannabinoids have been extensively studied for their potential anticancer effects and symptom management in cancer patients [7-9]. One of the first studies describing antineoplastic activity of cannabinoids was published in 1975 [10]. Potential antitumor activity of plant-derived or phytocannabinoids, e.g., (−)-trans-∆9-tetrahydrocannabinol (THC), cannabinol (CBN), ∆8-THC, cannabidiol (CBD) and cannabicyclol (CBL), as well as of synthetic cannabinoids, such as WIN-55,212-2, is the focus of current research [7,8,11].
In the 1990s, the main components of the endocannabinoid system (ECS) were identified as follows: (i) two types of cannabinoid (CB) receptors, CB1 and CB2 receptor; (ii) two main endogenous ligands (endocannabinoids) in mammals, anandamide or N-arachidonoyl ethanolamine (AEA) and 2-arachidonoylglycerol (2-AG); and (iii) endocannabinoid metabolic enzymes, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAG lipase). FAAH is the primary catabolic enzyme for fatty acid amides (FAAs), a class of bioactive lipids including AEA, while MAG lipase is a key enzyme in the hydrolysis of 2-AG [12-16]. Subsequent studies demonstrated the important role of the ECS and endocannabinoids in different physiological and pathological processes, such the regulation of excitatory and inhibitory synaptic transmission in the central nervous system (CNS), food intake, nociceptive signaling, analgesia, immunomodulation, inflammation, and cancer cell signaling [17-19].
In cancer patients, cannabinoids have primarily been used as a part of palliative care to alleviate pain, relieve nausea and stimulate appetite [8,20]. In addition, numerous cell culture and animal studies showed antitumor effects of cannabinoids and suggested new therapeutic opportunities for cancer patients [20]. However, recent research also emphasizes the importance of safety measures when using cannabinoids, since these compounds can potentially impair cognitive functions, especially in adolescents [21].
The aim of this article is to review the relevant literature on anticancer effects of plant-derived and synthetic cannabinoids, to increase our understanding of their potential mechanisms of action and possible role in cancer treatment. We also reviewed the current legislative updates on the use of cannabinoids for medical and therapeutic purposes, primarily in the EU countries.
MOLECULAR BASIS FOR CANNABINOID TREATMENT OF CANCER
The role of the endocannabinoid system in cancer
Endocannabinoids interact with different types of receptors, including the two Gi/o-coupled CB receptors, CB1 and CB2 [18]. While CB1 receptors are mainly located in the CNS and, to a lesser degree, in some peripheral tissues, CB2 receptors are primarily expressed on the surface of immune cells [22]. Due to the low expression of CB2 receptors in the CNS they represent a promising pharmacological target, as selective CB2 ligands potentially would not have psychotropic effects [23]. In addition, other CB receptor types and isoforms or completely different pharmacological targets of cannabinoids have been described, for example transient receptor potential vanilloid receptor 1 (TRPV1), orphan G-protein coupled receptor (GPR)55, peroxisome proliferator-activated receptors (PPARs) [24,25], transient receptor potential melastatin 8 (TRPM8), TRP vanilloid 2 (TRPV2) and TRP ankyrin 1 (TRPA1) channel [26]. It is important to note that cannabinoids may also exert their antitumor effects independent of the CB receptors, for example as demonstrated in human pancreatic cancer cell line MIA PaCa-2 [27].
The biological role of the ECS in cancer pathophysiology is not completely clear [20] but most studies suggest that CB receptors and their endogenous ligands are upregulated in tumor tissue [28,29,31,34-39,41,48] and that the overexpression of ECS components (i.e., receptors, ligands, and enzymes) correlates with tumor aggressiveness [49-51]. However, a tumor-suppressive role of ECS was also indicated by some studies, e.g., the upregulation of endocannabinoid-degrading enzymes was observed in aggressive human cancers and cancer cell lines [51]. Moreover, experimental studies showed that the activation of CB receptors by cannabinoids is antitumorigenic in most cases, i.e., it inhibits tumor cell proliferation, induces apoptosis in vitro, and blocks angiogenesis and tumor invasion/metastasis in vivo [35,46,51,52]. The effects of CB receptor (over)expression in selected human tumor cell lines are described in more detail in Table 1.
TABLE 1.
Expression of cannabinoid (CB) receptors in selected human cancer types

Antitumor effects of cannabinoids
By targeting the ECS, cannabinoids affect many essential cellular processes and signaling pathways which are crucial for tumor development [51,53,54]. For example, they can induce cell cycle arrest, promote apoptosis, and inhibit proliferation, migration and angiogenesis in tumor cells (Figure 1) [53,54]. In addition to CB receptor-mediated (CB1 and CB2 receptors) cannabinoid effects, it appears that these processes can also be CB receptor-independent (e.g., through TRPV1, 5-hydroxytryptamine [5-HT]3, or nicotinic acetylcholine receptor [nAChR] among others) [53], suggesting that molecular mechanisms underlying the antitumor activity of cannabinoids are even more complex than originally thought. Moreover, it is expected that future studies will discover novel molecular targets of cannabinoids [53].
FIGURE 1.
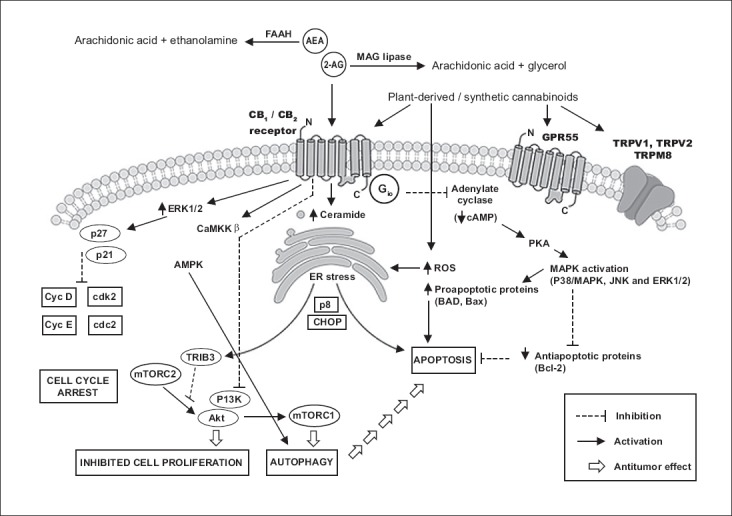
Example of different signaling pathways induced by cannabinoids in cancer cells [46,51,53-55]. By targeting the endocannabinoid system (ECS), cannabinoids affect many essential cellular processes and signaling pathways which are crucial for tumor development. For example, they can induce cell cycle arrest, promote apoptosis, and inhibit proliferation, migration and angiogenesis in tumor cells. AEA: Anandamide; 2-AG: 2-Arachidonoylglycerol; Akt: Protein Kinase B; AMPK: 5’ adenosine monophosphate-activated protein kinase; Bad: Bcl-2-associated death promoter; Bax: Apoptosis regulator; CaMKK: Calcium/calmodulin-dependent protein kinase kinase; Cdk 2: Cyclin-dependent kinase 2; CHOP: C/EBP homologous protein; CycD: Cyclin D; Cyc E: Cyclin E; ELK1: ETS domain-containing protein; ERK: Extracellular-signal-regulated kinase; FAAH: Fatty acid amide hydrolase; GPR55: Orphan G-protein coupled receptor 55; MAG lipase: Monoacylglycerol lipase; MAPK: Mitogen-activated protein kinase; p8: Candidate of metastasis 1; p21: Cyclin-dependent kinase inhibitor 1; p27: Cyclin-dependent kinase inhibitor 1B; PI3K: Phosphoinositide 3-kinase; PKA: Protein kinase A; ROS: Reactive oxygen species; TRPV1: Transient receptor potential vanilloid receptor 1; TRPV2: Transient receptor potential vanilloid receptor 2; TRPM8: Transient receptor potential melastatin 8; mTORC1: Mammalian target of rapamycin complex 1; mTORC2: Mammalian target of rapamycin complex 2; TRIB3: Tribbles homolog 3.
The ability of plant-derived and synthetic cannabinoids to control cancer cell growth, invasion, and death has been demonstrated in numerous experimental studies using cancer cell lines and genetically engineered mouse models. Also, different types of cannabinoids may have different modes of action. For example, a phytocannabinoid THC promotes apoptosis in a CB-receptor dependent manner, while CBD exerts this effect independently of CB1/CB2 receptors and possibly includes the activation of TRPV2 receptor, at least in some cancer types. Also, some CB receptor agonists are less efficient in promoting cancer cell death although they demonstrate higher affinity for CB receptors than THC, such as synthetic CB receptor agonist WIN-55,212-2. Better understanding of homo- or hetero-oligomerization of CB receptors, their interactions with lipid rafts for example, and mechanisms of selective G-protein coupling may clarify these differences [54]. Finally, because molecular changes are tumor-specific in most cases (i.e., the presence of intra- and inter-tumor heterogeneity), CB-receptor mediated antitumor effects largely depend on the type of cancer that is being investigated and characteristics of derived tumor cell line, including the donor characteristics, tumor site of origin and hormonal responsiveness [53-55].
PLANT-DERIVED CANNABINOIDS AND THEIR ANTITUMOR ACTIVITY
Phytocannabinoids are a group of C21 terpenophenolic compounds predominately produced by the plants from the genus Cannabis. Different resources indicate that there are more than 90 different cannabinoids together with their breakdown products, although some report that > 60 compounds is a more accurate estimation. Among these, the most abundant are THC, CBD, CBN and cannabichromene (CBC) followed by ∆8-THC, cannabidiolic acid (CBDA), cannabidivarin (CBDV) and cannabigerol (CBG). The highest content of cannabinoids is located in the flowering tops of the plant and small, young leaves around the flowers [56].
Pharmacologically, THC is a partial agonist at CB1 and CB2 receptor with inhibitory constant (Ki) of 40.7 nM for CB1 and 36.4 nM for CB2 [57]. ∆8-THC is a stable isomer of THC with similar Ki [58]. The most studied non-psychotropic phytocannabinoid is CBD which does not have psychotomimetic activity. CBD has a low affinity for CB1 and CB2; it was suggested that it acts as an antagonist of CB1/CB2 agonists but also as a CB2 inverse agonist (an inverse agonist binds to the same receptor-binding site as an agonist and it does not only antagonize the effects of the agonist but exerts the opposite effect). Other mechanisms of action of CBD, that are independent of CB receptors, include FAAH inhibition, inhibition of AEA reuptake, it acts as an agonist at PPARγ, TRPV1, TRPA1 and an antagonist at GPR55 and TRPM8 (Table 2). CBN is a weak partial agonist at CB1 (Ki of 308 nM) and CB2 (Ki of 96.3 nM); CBG is a potent TRPM8 antagonist, TRPV1 and TRPA1 agonist, and CB partial agonist; while CBC is a potent TRPA1 agonist and weak inhibitor of AEA reuptake [59].
TABLE 2.
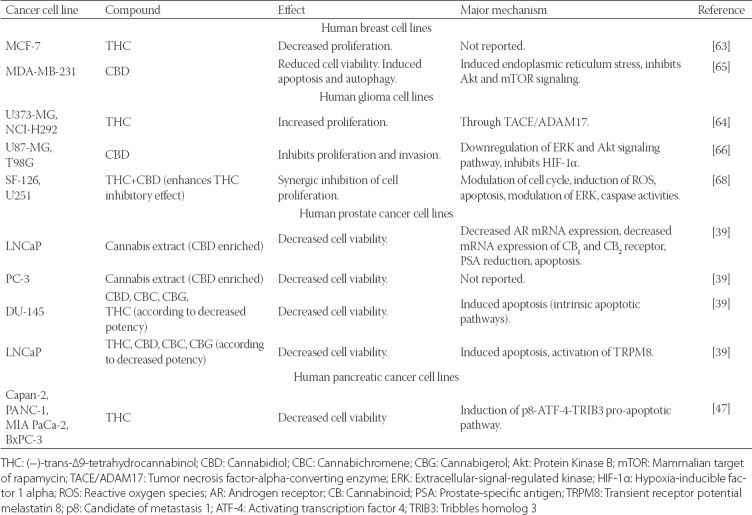
Antitumor activity of selected plant-derived cannabinoids in different cancer cell lines
Plant-derived cannabinoids are approved only for some indications, but additionally have been used off-label. For example, a standardized alcoholic cannabis extract nabiximols, which has the THC: CBD ratio of 1:1 and is available as an oromucosal spray, was approved in Germany for the treatment of moderate to severe refractory spasticity in multiple sclerosis. Examples of off-label use of this medication are of chronic pain in several medical conditions and symptomatic treatment of selected neuropsychological disorders (e.g., anxiety and sleeping disturbances). Common side effects of cannabinoids are tiredness and dizziness (in more than 10% of patients), dry mouth, and psychoactive effects among others. Nevertheless, tolerance to these side effects develops within a short time in almost all cases. Withdrawal symptoms are rarely observed in the therapeutic setting [60].
An exciting area of research is the technological improvement of existing pharmaceutical formulations, especially the development of new cannabis-based extracts. Romano et al. [57] found that a CO2 extracted cannabis extract, with a high content (64.8%) in Δ9-tetrahydrocannabivarin (THCV), inhibits nitrite production induced by lipopolysaccharides (LPS) in murine peritoneal macrophages, and thus may have a potential to modulate the inflammatory response in different disease conditions [57]. Another study compared in vitro antioxidant activity and gene expression of antioxidant enzymes between ethanol and supercritical fluid (SF) extracts of dehulled hemp seed. SF extract exhibited higher radical scavenging activities compared to ethanol extract. Both extracts upregulated the expression of the antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) in human hepatoma (HepG2) cells challenged with H2O2, and this effect was greater for SF extracts at the concentration of 500 µg/mL [61].
Different plant-derived cannabinoids and cannabis-based pharmaceutical drugs have been the subject of intensive research for their potential antitumor activity, especially in cancer cells that overexpress CB1 and/or CB2 receptors compared to normal tissues [62]. Many studies were conducted in different cell lines with cannabis extracts or individual isolated compounds and the results are sometimes confounding, because efficient anticancer effects, such as decreased proliferation of cancer cells, activation of apoptosis, inhibition of cell migration and decreased tumor vascularization are mainly recorded in breast, prostate and glioma cancer cell lines. In contrast, protumorigenic activity of natural cannabinoids, i.e., increased cell proliferation, has been reported in lung, breast, and hepatoma cell lines [63]. It appears that the balance between protumorigenic and antitumor effects of cannabinoids critically depends on their concentration, among other factors. For example, Hart et al. [64] showed that the treatment of glioblastoma U373-MG and lung carcinoma NCI-H292 cell line with nanomolar concentrations of THC (instead of commonly used micromoral concentrations) led to increased cell proliferation. The authors also emphasized that nanomolar concentrations of THC are more likely to be detected in the serum of patients after drug treatment [64]. Therefore, in cancer therapy, it is very important to consider the risk of acceleration of tumor growth due to the concentration-dependent proliferative potential of cannabinoids [64].
In addition to THC, CBD is another plant-derived cannabinoid that has been extensively studied for its potential antitumor effects [39,65-68]. In a panel of human prostate cancer cell lines, Sharma et al. [67] showed that CBD is a potent inhibitor of cancer cell growth, while this potency was significantly lower in non-cancer cells. Moreover, CBD downregulated CB1, CB2, vascular endothelial growth factor (VEGF) and prostate-specific antigen (PSA) in prostate cancer cells, as well as pro-inflammatory interleukin (IL)-6 and IL-8 in LPS-stimulated dermal fibroblasts, suggesting its anti-inflammatory properties [67]. Other studies showed that CBD preferentially inhibited the survival of breast cancer cells by inducing apoptosis and autophagy [65] and inhibited proliferation and cell invasion in human glioma cell lines [66].
The expression of CB1 and CB2 receptors on immune cells suggests their important role in the regulation of the immune system. Recently, it was demonstrated that the administration of THC into mice induced apoptosis in T cells and dendritic cells, leading to immunosuppression. Several studies suggested that cannabinoids are able to suppress inflammatory responses by downregulating cytokine and chemokine production and upregulating T-regulatory cells. Similar results were obtained with endocannabinoids, i.e., the administration of these compounds or the use of inhibitors of enzymes that break down endocannabinoids had an immunosuppressive effect and resulted in the recovery from immune-mediated injury to organs, e.g., in the liver [69]. As indicated in previous paragraphs, cannabinoids were able to stimulate cell proliferation in in vitro and/or in vivo models of several types of cancer. For example, a treatment with THC in the mouse mammary carcinoma 4T1 expressing low levels of CB1 and CB2 led to enhanced growth of tumor and metastasis, due to the inhibition of the antitumor immune response, primarily via CB2. Moreover, THC led to an increased production of IL-4 and IL-10 in these mice, indicating that it suppresses the Th1 response by enhancing Th2-associated cytokines as confirmed by their microarray data (Th2-related genes were upregulated and Th1-related genes downregulated). Lastly, the injection of anti-IL-4 and anti-IL-10 monoclonal antibodies partially reversed the THC-induced suppression of the immune response [70]. In another study, THC promoted tumorigenicity in two weakly immunogenic murine lung cancer models by inhibiting their antitumor immunity; namely, the inhibitory cytokines IL-10 and transforming growth factor beta (TGF-β) were upregulated, while interferon gamma (IFN-γ) was downregulated at the tumor site and in the spleens of the mice treated with THC [71]. These findings suggest that THC could decrease tumor immunogenicity and promote tumor growth by inhibiting antitumor immunity, probably via CB2 receptor-mediated, cytokine-dependent pathway. Additional studies on the interactions between cannabinoids and immune cells will provide crucial data to improve the efficacy and safety of cannabinoid therapy in oncology [72].
SYNTHETIC CANNABINOIDS WITH POTENTIAL ANTITUMOR EFFECTS
Most synthetic cannabinoids, including dronabinol, nabilone, and synthetic CBD are CB1 and CB2 receptor ligands [73]. Studies in cells and animals show that they produce similar qualitative physiological, psychoactive, analgesic, anti-inflammatory, and anticancer effects to plant-derived cannabinoids, but they can be up to 100× more potent than THC [73,74]. Similar to naturally occurring cannabinoids, synthetic cannabinoid agonists also demonstrated anticancer effects in certain cancer cell lines in vitro [17,75]. Oil and alcohol-based drops or capsules of dronabinol and nabilone (synthetic THC) as well as synthetic CBD are approved to treat cytostatic-induced nausea/vomiting in cancer patients and to stimulate appetite in patients with acquired immune deficiency syndrome [57].
Recently, a subclass of compounds emerged that act on metabolic enzymes involved in the regulation of ECS activity, such as inhibitors of FAAH which increase the levels of endogenous cannabinoid AEA. They were developed with the purpose to treat a variety of neurological diseases, chronic pain, obesity, and cancer [76]. A study investigating the combination of the synthetic analogue of AEA Met-F-AEA and the selective irreversible carbamate-based FAAH inhibitor URB597 showed that they synergistically inhibited epidermal growth factor (EGF)-induced proliferative and chemotactic activity of non-small cell lung cancer cell lines A549 and H460 [77]. Moreover, the two FAAH inhibitors URB597 and arachidonoyl serotonin (AA-5HT) had antimetastatic effects on A549 lung cancer cell metastasis [78]. However, recently in France, the first-in-human phase I clinical trial of an experimental FAAH inhibitor BIA 10-2474, for neuropathic pain treatment, ended up tragically; one person died and other four had irreversible brain damage [79,80]. The magnetic resonance imaging (MRI) showed evidence of deep cerebral hemorrhage and necrosis in the affected patients [79]. Other clinical trials conducted on FAAH inhibitors are Merck’s MK-4409, Pfizer’s PF-04457845, and Vernalis’ V158866; no adverse effects were reported with these agents and they were considered safe in humans [79,81,82]. Thus, it could be speculated that the negative effects of BIA 10-2474 occurred because the drug may have interacted with a wrong and unexpected molecular target [79]. Nevertheless, no FAAH inhibitor is yet approved for therapeutic use.
To summarize, the antitumor effects of synthetic cannabinoids such as the inhibition of cell growth, viability, proliferation and invasion, enhanced apoptosis, and suppression of specific proinflammatory cytokines are generally similar to the antitumor effects of plant-derived cannabinoids. Moreover, synthetic cannabinoids have the potential to be even more selective and potent than their natural counterparts and, thus, represent a promising therapeutic approach [73,74].
INTERNATIONAL AND NATIONAL LEGAL BASIS FOR THE USE OF CANNABINOIDS
As the number of studies investigating the medical and therapeutic potential of cannabinoids has increased in recent years, it is necessary to change the legislation on the use, cultivation, and marketing of cannabinoids. This should, however, be done with extreme care. In the Republic of Slovenia, the legislator made a significant progress in this area in 2017, which will be elaborated below.
In the EU Member States, the basis for developing and passing the legislation on cannabinoid use is provided by international conventions, including: i) the United Nations Single Convention on Narcotic Drugs, 1961 [83] and the 1972 Protocol amending the Single Convention on Narcotic Drugs, ii) the Convention on Psychotropic Substances 1971 [83], and iii) the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances 1988 [83].
The United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances provides additional legal mechanisms for enforcing the 1961 Single Convention on Narcotic Drugs and the 1971 Convention on Psychotropic Substances. Much of the treaty is devoted to fighting organized crime, but it also prohibits possession of drugs for personal use saying that “Subject to its constitutional principles and the basic concepts of its legal system, each Party shall adopt such measures as may be necessary to establish as a criminal offence under its domestic law, when committed intentionally, the possession, purchase or cultivation of narcotic drugs or psychotropic substances for personal consumption contrary to the provisions of the Conventions”, and this includes the cultivation of opium poppy, coca bush and cannabis plant for the production of narcotic drugs [83].
The United Nations Single Convention on Narcotic Drugs, 1961 sets out four Schedules. Substances controlled by the state are set out in Schedule I and Schedule II, preparations in Schedule III, whereas Schedule IV defines drugs, such as heroin. The Single Convention’s Schedules range from most restrictive to least restrictive, as follows: Schedule IV, Schedule I, Schedule II, Schedule III. Cannabis, cannabis resin, extracts and tinctures are included in the Schedule IV of The Single Convention on Narcotic Drugs. Tetrahydrocannabinol (THC, synonym delta-9-THC) is included in the Schedule I of the Convention on Psychotropic Substances. Delta-9-THC and its stereoisomers, including dronabinol, are listed in the Addendum 2 to the Convention on Psychotropic Substances. Nabilone is not controlled under international law [84].
Under the EU regulatory framework, the subject matter is regulated by Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use [85]. Pursuant to the Article 3 of Directive 2001/83/EC, this Directive shall not apply to medicinal products prepared in a pharmacy in accordance with a medical prescription, medicinal products prepared in a pharmacy in accordance with the prescriptions of a pharmacopoeia, and medicinal products intended for research and development trials. This Directive also allowed the use of medicinal products for human use, intended to be placed on the market in the Member States and either prepared industrially or manufactured by a method involving an industrial process. This made cannabinoid-based medicinal products available in all the Member States, provided they are permitted by the national legislation [84].
In the Republic of Slovenia, illicit drugs including cannabis, are governed by the following regulations: i) Production of and Trade in Illicit Drugs Act [86], ii) Act Regulating the Prevention of the Use of Illicit Drugs and the Treatment of Drug Users [87], iii) Criminal Code of the Republic of Slovenia [88], iv) Decree on the classification of illicit drugs [89], v) Rules on method and form of record-keeping and of reports on illicit drugs [90], and vi) the Rules governing the procedures for the issue of licenses for illicit drugs marketing [91].
As previously mentioned, in 2017 the adoption of the Decree amending the Decree on the classification of illicit drugs [92] was made. This Decree removed cannabis from Schedule I and placed it under Schedule II, with the note that the use of cannabis for medicinal purposes is permitted in accordance with the Medicinal Products Act [93] and Pharmacy Services Act [94], and in accordance with the rules and regulations governing the prescribing of cannabinoid-based drugs.
The aforementioned amendment to the Decree on the classification of illicit drugs now allows patients to use medicinal cannabis as a means of treatment, including the cannabis plant and cannabis resin. Medicinal products are thus not limited anymore to products containing nabilone or cannabis extracts, but also extend to tinctures adjusted and harmonized to delta-9-THC, as long as they meet the conditions laid down in the Medicinal Products Act.
Changes in the legislation on the use of cannabinoids for medical purposes and inclusion of these compounds in the list of medicinal products needs to be coordinated with the changes in both labor law and the regulation of workplace drug testing. Naturally, any change should be adopted in strict agreement with work, health, and safety regulations and ensure smooth workflow for the employees.
CONCLUSION
Cannabinoids are a large and important class of complex compounds that have a promising therapeutic potential for the treatment of variety of diseases, including cancer. In this review, we focused on studies that provided evidence for anticancer effects of plant-derived and synthetic cannabinoids and their potential mechanisms of action. Cannabinoids were able to effectively modulate tumor growth in different in vitro and in vivo cancer models, however, these anticancer effects appears to be dependent on cancer type and drug dose. Understanding how cannabinoids are able to modulate essential cellular processes involved in tumorigenesis, such as the progression through the cell cycle, cell proliferation and cell death, as well as the interactions between cannabinoids and immune system are crucial for improving existing medications and developing new therapeutic approaches.
Although still strict, the legislation on the use of cannabis-based medications has been improved, especially following the promising results of related basic research. The Republic of Slovenia established a legal basis for the use of cannabinoids in the years 2016 and 2017. The increasing popularity of cannabis and cannabis-based medication should lead to clear regulatory guidelines on their use, in the near future.
ACKNOWLEDGMENTS
The authors acknowledge Jan Schmidt for his initial help in preparing this manuscript.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Touw M. The religious and medicinal uses of cannabis in China, India and Tibet. J Psychoactive Drugs. 1981;13(1):23–34. doi: 10.1080/02791072.1981.10471447. https://doi.org/10.1080/02791072.1981.10471447. [DOI] [PubMed] [Google Scholar]
- 2.Robinson SM, Adinoff B. The classification of substance use disorders:Historical, contextual, and conceptual considerations. Behav Sci (Basel) 2016;6(3):18. doi: 10.3390/bs6030018. https://doi.org/10.3390/bs6030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuardi AW. History of cannabis as a medicine:A review. Rev Bras Psiquiatr. 2006;28(2):153–7. doi: 10.1590/s1516-44462006000200015. https://doi.org/10.1590/S1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
- 4.American Herbal Pharmacopoeia®[Internet] Scotts Valley: American Herbal Pharmacopoeia®; 2018. [[cited 2018 May 30]]. Available from: http://www.herbal-ahp.org/ [Google Scholar]
- 5.Bifulco M, Pisanti S. Medicinal use of cannabis in Europe:The fact that more countries legalize the medicinal use of cannabis should not become an argument for unfettered and uncontrolled use. EMBO Rep. 2015;16(2):130–2. doi: 10.15252/embr.201439742. https://doi.org/10.15252/embr.201439742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko GD, Bober SL, Mindra S, Moreau JM. Medical cannabis - The Canadian perspective. J Pain Res. 2016;9:735–44. doi: 10.2147/JPR.S98182. https://doi.org/10.2147/JPR.S98182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birdsall SM, Birdsall TC, Tims LA. The use of medical marijuana in cancer. Curr Oncol Rep. 2016;18(7):40. doi: 10.1007/s11912-016-0530-0. https://doi.org/10.1007/s11912-016-0530-0. [DOI] [PubMed] [Google Scholar]
- 8.Davis MP. Cannabinoids for symptom management and cancer therapy:The evidence. J Natl Compr Canc Netw. 2016;14(7):915–22. doi: 10.6004/jnccn.2016.0094. https://doi.org/10.6004/jnccn.2016.0094. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute [Internet] USA: National Cancer Institute; 2018. [[cited 2018 May 30]]. Available from: https://www.cancer.gov/about-cancer/understanding/statistics . [Google Scholar]
- 10.Munson AE, Harris LS, Friedman MA, Dewey WL, Carchman RA. Antineoplastic activity of cannabinoids. J Natl Cancer Inst. 1975;55(3):597–602. doi: 10.1093/jnci/55.3.597. https://doi.org/10.1093/jnci/55.3.597. [DOI] [PubMed] [Google Scholar]
- 11.Müller L, Radtke A, Decker J, Koch M, Belge G. The synthetic cannabinoid Win 55,212-2 elicits in human cancer cell lines. Anticancer Res. 2017;37(11):6341–5. doi: 10.21873/anticanres.12086. https://doi.org/10.21873/anticanres.12086. [DOI] [PubMed] [Google Scholar]
- 12.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–80. doi: 10.1016/s0163-7258(97)82001-3. https://doi.org/10.1016/S0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 13.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–9. doi: 10.1126/science.1470919. https://doi.org/10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 14.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–13. [PubMed] [Google Scholar]
- 15.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–5. doi: 10.1038/365061a0. https://doi.org/10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 16.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–32. doi: 10.1146/annurev.biochem.74.082803.133450. https://doi.org/10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 17.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8(6):403–21. doi: 10.2174/187152709789824660. https://doi.org/10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadolska K, Gos R. The role of endocannabinoid system in physiological and pathological processes in the eye. [Article in Polish] Klin Oczna. 2008;110(10-12):392–6. [PubMed] [Google Scholar]
- 19.Fogli S, Breschi MC. The molecular bases of cannabinoid action in cancer. Cancer Therapy. 2008;6:103–16. [Google Scholar]
- 20.Javid FA, Phillips RM, Afshinjavid S, Verde R, Ligresti A. Cannabinoid pharmacology in cancer research:A new hope for cancer patients? Eur J Pharmacol. 2016;775:1–14. doi: 10.1016/j.ejphar.2016.02.010. https://doi.org/10.1016/j.ejphar.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Skosnik PD, Cortes-Briones JA, Hajos M. It's all in the rhythm:The role of cannabinoids in neural oscillations and psychosis. Biol Psychiatry. 2016;79(7):568–77. doi: 10.1016/j.biopsych.2015.12.011. https://doi.org/10.1016/j.biopsych.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors:Nomenclature and pharmacological principles. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(1):4–15. doi: 10.1016/j.pnpbp.2012.02.009. https://doi.org/10.1016/j.pnpbp.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007;152(5):567–75. doi: 10.1038/sj.bjp.0707481. https://doi.org/10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morice AH, Geppetti P. Cough. 5:The type 1 vanilloid receptor:a sensory receptor for cough. Thorax. 2004;59(3):257–8. doi: 10.1136/thx.2003.013482. https://doi.org/10.1136/thx.2003.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latorre JG, Schmidt EB. Cannabis, cannabinoids, and cerebral metabolism:Potential applications in stroke and disorders of the central nervous system. Curr Cardiol Rep. 2015;17(9):627. doi: 10.1007/s11886-015-0627-3. https://doi.org/10.1007/s11886-015-0627-3. [DOI] [PubMed] [Google Scholar]
- 26.De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163(7):1479–94. doi: 10.1111/j.1476-5381.2010.01166.x. https://doi.org/10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogli S, Nieri P, Chicca A, Adinolfi B, Mariotti V, Iacopetti P, et al. Cannabinoid derivatives induce cell death in pancreatic MIA PaCa-2 cells via a receptor-independent mechanism. FEBS Lett. 2006;580(7):1733–9. doi: 10.1016/j.febslet.2006.02.024. https://doi.org/10.1016/j.febslet.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Gomez E, Andradas C, Blasco-Benito S, Caffarel MM, Garcia-Taboada E, Villa-Morales, et al. Role of cannabinoid receptor CB2 in HER2 pro-oncogenic signaling in breast cancer. J Natl Cancer Inst. 2015;107(6):djv077. doi: 10.1093/jnci/djv077. https://doi.org/10.1093/jnci/djv077. [DOI] [PubMed] [Google Scholar]
- 29.Caffarel MM, Andradas C, Mira E, Perez-Gomez E, Cerutti C, Moreno-Bueno G, et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer. 2010;9:196. doi: 10.1186/1476-4598-9-196. https://doi.org/10.1186/1476-4598-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vercelli C, Barbero R, Cuniberti B, Racca S, Abbadessa G, Piccione F, et al. Transient receptor potential vanilloid 1 expression and functionality in MCF-7 cells:A preliminary investigation. J Breast Cancer. 2014;17(4):332–8. doi: 10.4048/jbc.2014.17.4.332. https://doi.org/10.4048/jbc.2014.17.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung SC, Hammarsten P, Josefsson A, Stattin P, Granfors T, Egevad L, et al. A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur J Cancer. 2009;45(1):174–82. doi: 10.1016/j.ejca.2008.10.010. https://doi.org/10.1016/j.ejca.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Cipriano M, Haggstrom J, Hammarsten P, Fowler CJ. Association between cannabinoid CB1 receptor expression and Akt signalling in prostate cancer. PLoS One. 2013;8(6):e65798. doi: 10.1371/journal.pone.0065798. https://doi.org/10.1371/journal.pone.0065798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velasco G, Hernandez S, Davila D, Lorente M. The use of cannabinoids as anticancer agents. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:259–66. doi: 10.1016/j.pnpbp.2015.05.010. https://doi.org/10.1016/j.pnpbp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Thors L, Bergh A, Persson E, Hammarsten P, Stattin P, Egevad L, et al. Fatty acid amide hydrolase in prostate cancer:Association with disease severity and outcome, CB1 receptor expression and regulation by IL-4. PLoS One. 2010;5(8):e12275. doi: 10.1371/journal.pone.0012275. https://doi.org/10.1371/journal.pone.0012275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment:Progress and promise. Cancer Res. 2008;68(2):339–42. doi: 10.1158/0008-5472.CAN-07-2785. https://doi.org/10.1158/0008-5472.CAN-07-2785. [DOI] [PubMed] [Google Scholar]
- 36.Orellana-Serradell O, Poblete CE, Sanchez C, Castellon EA, Gallegos I, Huidobro C, et al. Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol Rep. 2015;33(4):1599–1608. doi: 10.3892/or.2015.3746. https://doi.org/10.3892/or.2015.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez MG, Sanchez AM, Collado B, Malagarie-Cazenave S, Olea N, Carmena MJ, et al. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. 2005;515(1-3):20–7. doi: 10.1016/j.ejphar.2005.04.010. https://doi.org/10.1016/j.ejphar.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Pecze L, Josvay K, Blum W, Petrovics G, Vizler C, Olah Z, et al. Activation of endogenous TRPV1 fails to induce overstimulation-based cytotoxicity in breast and prostate cancer cells but not in pain-sensing neurons. Biochim Biophys Acta. 2016;1863(8):2054–64. doi: 10.1016/j.bbamcr.2016.05.007. https://doi.org/10.1016/j.bbamcr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 39.De Petrocellis L, Ligresti A, Schiano Moriello A, Iappelli M, Verde R, Stott CG, et al. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo:Pro-apoptotic effects and underlying mechanisms. Br J Pharmacol. 2013;168(1):79–102. doi: 10.1111/j.1476-5381.2012.02027.x. https://doi.org/10.1111/j.1476-5381.2012.02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pineiro R, Maffucci T, Falasca M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene. 2011;30(2):142–52. doi: 10.1038/onc.2010.417. https://doi.org/10.1038/onc.2010.417. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay B, Schuebel K, Mukhopadhyay P, Cinar R, Godlewski G, Xiong K, et al. Cannabinoid receptor 1 promotes hepatocellular carcinoma initiation and progression through multiple mechanisms. Hepatology. 2015;61(5):1615–26. doi: 10.1002/hep.27686. https://doi.org/10.1002/hep.27686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X, Liu Y, Huang S, Liu G, Xie C, Zhou J, et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. 2006;171(1):31–8. doi: 10.1016/j.cancergencyto.2006.06.014. https://doi.org/10.1016/j.cancergencyto.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Preet A, Qamri Z, Nasser MW, Prasad A, Shilo K, Zou X, et al. Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev Res (Phila) 2011;4(1):65–75. doi: 10.1158/1940-6207.CAPR-10-0181. https://doi.org/10.1158/1940-6207.CAPR-10-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freund P, Porpaczy EA, Le T, Gruber M, Pausz C, Staber P, et al. Cannabinoid receptors are overexpressed in CLL but of limited potential for therapeutic exploitation. PLoS One. 2016;11(6):e0156693. doi: 10.1371/journal.pone.0156693. https://doi.org/10.1371/journal.pone.0156693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michalski CW, Oti FE, Erkan M, Sauliunaite D, Bergmann F, Pacher P, et al. Cannabinoids in pancreatic cancer:Correlation with survival and pain. Int J Cancer. 2008;122(4):742–50. doi: 10.1002/ijc.23114. https://doi.org/10.1002/ijc.23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakravarti B, Ravi J, Ganju RK. Cannabinoids as therapeutic agents in cancer:Current status and future implications. Oncotarget. 2014;5(15):5852–72. doi: 10.18632/oncotarget.2233. https://doi.org/10.18632/oncotarget.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carracedo A, Gironella M, Lorente M, Garcia S, Guzman M, Velasco G, et al. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 2006;66(13):6748–55. doi: 10.1158/0008-5472.CAN-06-0169. https://doi.org/10.1158/0008-5472.CAN-06-0169. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Z, Yang J, Zhao H, Fang X, Li H. Cannabinoid receptor 2 is upregulated in melanoma. J Cancer Res Ther. 2012;8(4):549–54. doi: 10.4103/0973-1482.106534. https://doi.org/10.4103/0973-1482.106534. [DOI] [PubMed] [Google Scholar]
- 49.Malfitano AM, Ciaglia E, Gangemi G, Gazzerro P, Laezza C, Bifulco M. Update on the endocannabinoid system as an anticancer target. Expert Opin Ther Targets. 2011;15(3):297–308. doi: 10.1517/14728222.2011.553606. https://doi.org/10.1517/14728222.2011.553606. [DOI] [PubMed] [Google Scholar]
- 50.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140(1):49–61. doi: 10.1016/j.cell.2009.11.027. https://doi.org/10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velasco G, Sanchez C, Guzman M. Endocannabinoids and cancer. Handb Exp Pharmacol. 2015;231:449–72. doi: 10.1007/978-3-319-20825-1_16. https://doi.org/10.1007/978-3-319-20825-1_16. [DOI] [PubMed] [Google Scholar]
- 52.Guzman M. Cannabinoids:Potential anticancer agents. Nat Rev Cancer. 2003;3(10):745–55. doi: 10.1038/nrc1188. https://doi.org/10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 53.Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78(6):549–63. doi: 10.1016/j.lfs.2005.05.055. https://doi.org/10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 54.Velasco G, Sanchez C, Guzman M. Anticancer mechanisms of cannabinoids. Curr Oncol. 2016;23(Suppl 2):S23–S32. doi: 10.3747/co.23.3080. https://doi.org/10.3747/co.23.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caffarel MM, Andradas C, Perez-Gomez E, Guzman M, Sanchez C. Cannabinoids:A new hope for breast cancer therapy? Cancer Treat Rev. 2012;38(7):911–8. doi: 10.1016/j.ctrv.2012.06.005. https://doi.org/10.1016/j.ctrv.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Andre CM, Hausman JF, Guerriero G. Cannabis sativa:The plant of the thousand and one molecules. Front Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. https://doi.org/10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romano B, Pagano E, Orlando P, Capasso R, Cascio MG, Pertwee R, et al. Pure delta9-tetrahydrocannabivarin and a Cannabis sativa extract with high content in delta9-tetrahydrocannabivarin inhibit nitrite production in murine peritoneal macrophages. Pharmacol Res. 2016;113(Pt A):199–208. doi: 10.1016/j.phrs.2016.07.045. https://doi.org/10.1016/j.phrs.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 58.Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2):Identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278(3):989–99. [PubMed] [Google Scholar]
- 59.Izzo AA, Borrell F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids:New therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):515–27. doi: 10.1016/j.tips.2009.07.006. https://doi.org/10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Grotenhermen F, Muller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Dtsch Arztebl Int. 2012;109(29-30):495–501. doi: 10.3238/arztebl.2012.0495. https://doi.org/10.3238/arztebl.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong S, Sowndhararajan K, Joo T, Lim C, Cho H, Kim S, et al. Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells. Asian Pac J Reprod. 2015;4(2):147–52. https://doi.org/10.1016/S2305-0500(15)30012-9. [Google Scholar]
- 62.McAllister SD, Soroceanu L, Desprez PY. The antitumor activity of plant-derived non-psychoactive cannabinoids. J Neuroimmune Pharmacol. 2015;10(2):255–67. doi: 10.1007/s11481-015-9608-y. https://doi.org/10.1007/s11481-015-9608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cridge BJ, Rosengren RJ. Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag Res. 2013;5:301–13. doi: 10.2147/CMAR.S36105. https://doi.org/10.2147/CMAR.S36105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hart S, Fischer OM, Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004;64(6):1943–50. doi: 10.1158/0008-5472.can-03-3720. https://doi.org/10.1158/0008-5472.CAN-03-3720. [DOI] [PubMed] [Google Scholar]
- 65.Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10(7):1161–72. doi: 10.1158/1535-7163.MCT-10-1100. https://doi.org/10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- 66.Solinas M, Massi P, Cinquina V, Valenti M, Bolognini D, Gariboldi M, et al. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS One. 2013;8(10):e76918. doi: 10.1371/journal.pone.0076918. https://doi.org/10.1371/journal.pone.0076918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma M, Hudson J, Adomat H, Guns E, Cox M. In vitro anticancer activity of plant-derived cannabidiol on prostate cancer cell lines. Pharmacol Pharm. 2014;5(8):806–20. https://doi.org/10.4236/pp.2014.58091. [Google Scholar]
- 68.Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, Lee J, et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol Cancer Ther. 2010;9(1):180–9. doi: 10.1158/1535-7163.MCT-09-0407. https://doi.org/10.1158/1535-7163.MCT-09-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1(7):1333–49. doi: 10.4155/fmc.09.93. https://doi.org/10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKallip RJ, Nagarkatti M, Nagarkatti PS. Δ-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol. 2005;174(6):3281–9. doi: 10.4049/jimmunol.174.6.3281. https://doi.org/10.4049/jimmunol.174.6.3281. [DOI] [PubMed] [Google Scholar]
- 71.Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP, et al. Δ-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol. 2000;165(1):373–80. doi: 10.4049/jimmunol.165.1.373. https://doi.org/10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]
- 72.Hanlon KE, Lozano-Ondoua AN, Umaretiya PJ, Symons-Liguori AM, Chandramouli A, Moy JK, et al. Modulation of breast cancer cell viability by a cannabinoid receptor 2 agonist, JWH-015, is calcium dependent. Breast Cancer (Dove Med Press) 2016;8:59–71. doi: 10.2147/BCTT.S100393. https://doi.org/10.2147/BCTT.S100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA. Synthetic cannabinoids:Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend 2014; 144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005. https://doi.org/10.1016/j.drugalcdep.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Debruyne D, Le Boisselier R. Emerging drugs of abuse:Current perspectives on synthetic cannabinoids. Subst Abuse Rehabil. 2015;6:113–29. doi: 10.2147/SAR.S73586. https://doi.org/10.2147/SAR.S73586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guzman M, Sanchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol Ther 2002; 95(2):175–84. doi: 10.1016/s0163-7258(02)00256-5. https://doi.org/10.1016/S0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 76.Otrubova K, Ezzili C, Boger DL. The discovery and development of inhibitors of fatty acid amide hydrolase (FAAH) Bioorg Med Chem Lett. 2011;21(16):4674–85. doi: 10.1016/j.bmcl.2011.06.096. https://doi.org/10.1016/j.bmcl.2011.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ravi J, Sneh A, Shilo K, Nasser MW, Ganju RK. FAAH inhibition enhances anandamide mediated anti-tumorigenic effects in non-small cell lung cancer by downregulating the EGF/EGFR pathway. Oncotarget. 2014;5(9):2475–86. doi: 10.18632/oncotarget.1723. https://doi.org/10.18632/oncotarget.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winkler K, Ramer R, Dithmer S, Ivanov I, Merkord J, Hinz B. Fatty acid amide hydrolase inhibitors confer anti-invasive and antimetastatic effects on lung cancer cells. Oncotarget. 2016;7(12):15047–64. doi: 10.18632/oncotarget.7592. https://doi.org/10.18632/oncotarget.7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaur R, Sidhu P, Singh S. What failed BIA 10-2474 phase I clinical trial?Global speculations and recommendations for future Phase I trials. J Pharmacol Pharmacother. 2016;7(3):120–6. doi: 10.4103/0976-500X.189661. https://doi.org/10.4103/0976-500X.189661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Q. The price of mistakes in clinical trials [Internet] NY: The Rockefeller university; 2012. Jun 1, [[cited on:2018 May 30]]. Available from: http://selections.rockefeller.edu/the-price-of-mistakes-in-clinical-trials/ [Google Scholar]
- 81.U.S. National Library of Medicine [Internet] Bethesda: U.S. National Library of Medicine; 2018. [[cited on:2018 May 30]]. Available from https://www.clinicaltrials.gov/ct2/show/NCT00981357 . [Google Scholar]
- 82.U.S. National Library of Medicine [Internet] Bethesda: U.S. National Library of Medicine; 2018. [[cited on:2018 May 30]]. Available from https://www.clinicaltrials.gov/ct2/show/NCT01748695 . [Google Scholar]
- 83.Slovenia. International Treaties. Act notifying the succession of the Republic of Slovenia to United Nations conventions and conventions adopted by the International Atomic Energy Agency. No 9/92. Ljubljana: Ministry of Justice; 1992. [Google Scholar]
- 84.Čufar A. Prescribing of cannabinoids –A regulatory view. Pharmaceutical Journal of Slovenia. 2016;67:91–5. [Google Scholar]
- 85.European Union. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Brussels: European Parliament; 2011. [Google Scholar]
- 86.Slovenia. Production of and Trade in Illicit Drugs Act. No 47/04. Ljubljana: Ministry of Justice; 2004. [Google Scholar]
- 87.Slovenia. Act Regulating the Prevention of the Use of Illicit Drugs and the Treatment of Drug Users. No 2/04. Ljubljana: Ministry of Justice; 2004. [Google Scholar]
- 88.Slovenia. Criminal Code of the Republic of Slovenia. No 38/2016. Ljubljana: Ministry of Justice; 2016. [Google Scholar]
- 89.Slovenia. Decree on the classification of illicit drugs. No 14/2017. Ljubljana: Ministry of Justice; 2017. [Google Scholar]
- 90.Slovenia. Rules on method and form of record-keeping and of reports on illicit drugs. No 131/2006. Ljubljana: Ministry of Justice; 2006. [Google Scholar]
- 91.Slovenia. Rules governing the procedures for the issue of licenses for illicit drugs marketing. No 8/2002. Ljubljana: Ministry of Justice; 2002. [Google Scholar]
- 92.Slovenia. Decree amending the Decree on the classification of illicit drugs. No 14/2017. Ljubljana: Ministry of Justice; 2017. [Google Scholar]
- 93.Slovenia. Medicinal Products Act. No 17/2014. Ljubljana: Ministry of Health; 2014. [Google Scholar]
- 94.Slovenia. Pharmacy Services Act. No 85/2016. Ljubljana: Ministry of Health; 2016. [Google Scholar]


