Summary
Background
Avelumab (MSB0010718C) is a human IgG1 monoclonal antibody that binds to PD-L1, inhibiting its binding to PD-1, which inactivates T cells. We aimed to establish the safety and pharmacokinetics of avelumab in patients with solid tumours while assessing biological correlatives for future development.
Methods
This open-label, single-centre, phase 1a, dose-escalation trial (part of the JAVELIN Solid Tumor trial) assessed four doses of avelumab (1 mg/kg, 3 mg/kg, 10 mg/kg, and 20 mg/kg), with dose-level cohort expansions to provide additional safety, pharmacokinetics, and target occupancy data. This study used a standard 3 + 3 cohort design and assigned patients sequentially at trial entry according to the 3 + 3 dose-escalation algorithm and depending on the number of dose-limiting toxicities during the first 3-week assessment period (the primary endpoint). Patient eligibility criteria included age 18 years or older, Eastern Cooperative Oncology Group performance status 0–1, metastatic or locally advanced previously treated solid tumours, and adequate end-organ function. Avelumab was given as a 1-h intravenous infusion every 2 weeks. Patients in the dose-limiting toxicity analysis set were assessed for the primary endpoint of dose-limiting toxicity, and all patients enrolled in the dose-escalation part were assessed for the secondary endpoints of safety (treatment-emergent and treatment-related adverse events according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0), pharmacokinetic and pharmacodynamic profiles (immunological effects), best overall response by Response Evaluation Criteria, and antidrug antibody formation. The population for the pharmacokinetic analysis included a subset of patients with rich pharmacokinetic samples from two selected disease-specific expansion cohorts at the same study site who had serum samples obtained at multiple early timepoints. This trial is registered with ClinicalTrials.gov, number NCT01772004. Patient recruitment to the dose-escalation part reported here is closed.
Findings
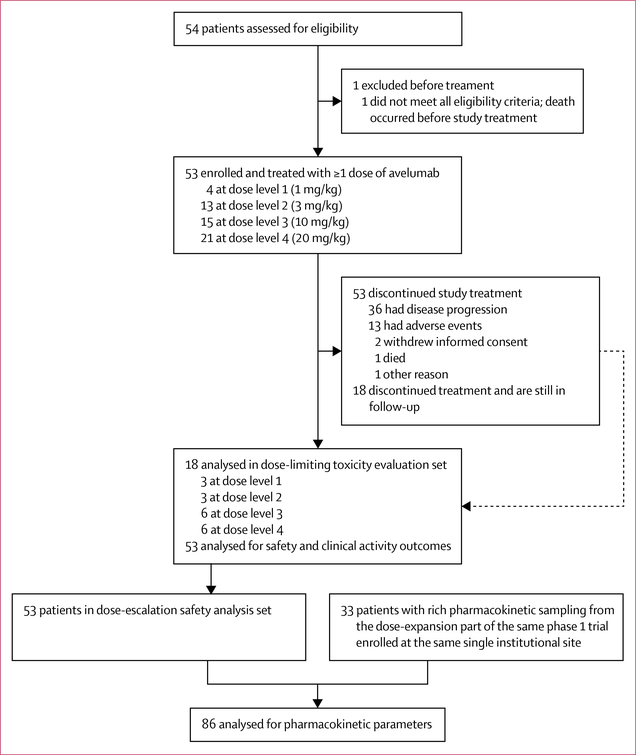
Between Jan 31, 2013, and Oct 8, 2014, 53 patients were enrolled (four patients at 1 mg/kg, 13 at 3 mg/kg, 15 at 10 mg/kg, and 21 at 20 mg/kg). 18 patients were analysed in the dose-limiting toxicity analysis set: three at dose level 1 (1 mg/kg), three at dose level 2 (3 mg/kg), six at dose level 3 (10 mg/kg), and six at dose level 4 (20 mg/kg). Only one dose-limiting toxicity occurred, at the 20 mg/kg dose, and thus the maximum tolerated dose was not reached. In all 53 enrolled patients (the safety analysis set), common treatment-related adverse events (occurring in >10% of patients) included fatigue (21 patients [40%]), influenza-like symptoms (11 [21%]), fever (8 [15%]), and chills (6 [11%]). Grade 3–4 treatment-related adverse events occurred in nine (17%) of 53 patients, with autoimmune disorder (n=3), increased blood creatine phosphokinase (n=2), and increased aspartate aminotransferase (n=2) each occurring in more than one patient (autoimmune disorder in two patients at 10 mg/kg and one patient at 20 mg/kg, increased blood creatine phosphokinase in two patients at 20 mg/kg, and increased aspartate aminotransferase in one patient at 1 mg/kg, and one patient at 10 mg/kg). Six (11%) of 53 patients had a serious treatment-related adverse event: autoimmune disorder (two [13%]), lower abdominal pain (one [7%]), fatigue (one [7%]), and influenza-like illness (one [7%]) in three patients treated at 10 mg/kg dose level, and autoimmune disorder (one [5%]), increased amylase (one [5%]), myositis (one [5%]), and dysphonia (one [5%]) in three patients who received the 20 mg/kg dose. We recorded some evidence of clinical activity in various solid tumours, with partial confirmed or unconfirmed responses in four (8%) of 53 patients; 30 (57%) additional patients had stable disease. Pharmacokinetic analysis (n=86) showed a dose-proportional exposure between doses of 3 mg/kg and 20 mg/kg and a half-life of 95–99 h (3·9–4·1 days) at the 10 mg/kg and 20 mg/kg doses. Target occupancy was greater than 90% at doses of 3 mg/kg and 10 mg/kg. Antidrug antibodies were detected in two (4%) of 53 patients. No substantial differences were found in absolute lymphocyte count or multiple immune cell subsets, including those expressing PD-L1, after treatment with avelumab. 31 (58%) of 53 patients in the overall safety population died; no deaths were related to treatment on study.
Interpretation
Avelumab has an acceptable toxicity profile up to 20 mg/kg and the maximum tolerated dose was not reached. Based on pharmacokinetics, target occupancy, and immunological analysis, we chose 10 mg/kg every 2 weeks as the dose for further development and phase 3 trials are ongoing.
Introduction
Avelumab (MSB0010718C) is a fully human immunoglobulin G1 (IgG1) monoclonal antibody that specifically binds PD-L1 and inhibits its binding to PD-1, which otherwise results in T-cell inactivation and suppression. Monoclonal antibodies targeting PD-1 and PD-L1 have shown antitumour activity in a range of human solid tumours, including melanoma and cancers of the lung, kidney, head and neck, bladder, stomach, and breast.1 Two antibodies specific for PD-1 (nivolumab and pembrolizumab) and two specific for PD-L1 (atezolizumab and avelumab) have been approved by the US Food and Drug Administration (FDA) for the treatment of several tumour types, including metastatic melanoma,2,3 non-small-cell lung cancer,4–7 bladder cancer,8 and metastatic Merkel cell carcinoma.9 Although other agents targeting PD-L1 are in clinical development, to the best of our knowledge, avelumab is the first human anti-PD-L1 IgG1 antibody with a native Fc region, which means it is capable of inducing antibody-dependent cell-mediated cytotoxicity (ADCC).10–12
Toxicology studies in cynomolgus monkeys determined a starting dose of 1 mg/kg for avelumab dose-escalation in human beings (Neuteboom B, unpublished). This starting dose level was further supported by preliminary pharmacokinetic data showing that this dose level produced serum concentrations associated with pharmacological activity (ie, target occupancy and T-cell activation; Neuteboom B, unpublished). Previous in-vitro studies have shown several key findings: avelumab can lyse a range of human tumour cells in the presence of peripheral blood mononuclear cells (PBMCs) or natural killer (NK) cells; interferon γ can enhance tumour cell PD-L1 expression and, in some cases, enhance ADCC tumour cell lysis; purified NK cells are potent effectors for avelumab; levels of avelumab-mediated ADCC lysis of tumour cells are similar using purified NK cells from either healthy donors or patients with cancer; levels of avelumab-mediated lysis are very low when whole PBMCs are used as targets; and the addition of interleukin 12 to NK cells greatly enhances avelumab-mediated ADCC.10,13 A recent in-vitro study14 has also shown that avelumab can lyse a residential cancer stem-cell population of chordoma cells via the ADCC mechanism. These studies thus provide evidence that avelumab, through its ADCC activity, might have an additional method of action for an anti-PD-L1 antibody. In this phase 1a study, we aimed to establish the safety and pharmacokinetics of avelumab and to assess the biological correlatives of different doses of avelumab. This paper reports the dose-escalation part of the trial; dose-expansion cohorts are reported separately.15
Methods
Study design and participants
The dose-escalation part of the JAVELIN Solid Tumor trial was an open-label, phase 1a study. All patients were enrolled at the Center for Cancer Research of the National Cancer Institute (Bethesda, MD, USA). Patient eligibility criteria included age 18 years or older, Eastern Cooperative Oncology Group performance status 0–1, life expectancy of at least 3 months, and histologically or cytologically confirmed metastatic or locally advanced solid tumours for which no standard therapy exists or standard therapy has failed. All patients, irrespective of solid tumour type, disease histology, and molecular biomarkers, had to have measurable disease by Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 and adequate end-organ function (defined by the following laboratory values: white blood cell count ≥3 × 109 cells per L with an absolute neutrophil count ≥1·5 × 109 cells per L, lymphocyte count ≥0·5 × 109 cells per L, platelet count ≥100 × 109 platelets per L, haemoglobin ≥9 g/dL, total bilirubin concentration of ≤1·5 × the upper limit of normal [ULN] range, aspartate aminotransferase and alanine aminotransferase concentrations of ≤2·5 × ULN), and an estimated creatinine clearance greater than 50 mL per min according to the Cockcroft-Gault formula. No previous use of a T-cell-targeting immune checkpoint inhibitor was allowed, and no other anticancer therapies could be continued on study, with the exception of androgen-deprivation therapy in metastatic castration-resistant prostate cancer or palliative bone-directed radiotherapy. Patients could not have had another cancer diagnosis within the previous 5 years, rapidly progressive disease, CNS metastases, a previous stem-cell or organ transplant, known hypersensitivity to monoclonal antibodies, or known autoimmune disease. Pregnant or lactating women were excluded because of the unknown effects of avelumab on a foetus or infant. Steroid use within 30 days of enrolment was not allowed, and steroids were prohibited on study except to manage autoimmune toxicity.
The study was done in accordance with the ethics principles of the Declaration of Helsinki and the International Council on Harmonisation Guidelines on Good Clinical Practice. The study protocol was reviewed and approved by the National Cancer Institute’s Institutional Review Board. Written informed consent was obtained from all study participants.
Procedures
We enrolled patients in a standard 3 + 3 dose-escalation phase to assess four dose levels (1 mg/kg bodyweight [dose level 1], 3 mg/kg bodyweight [dose level 2], 10 mg/kg bodyweight [dose level 3], and 20 mg/kg bodyweight [dose level 4]) and then expanded enrolment at the three highest doses (3 mg/kg, 10 mg/kg, and 20 mg/kg) to obtain supplemental data for safety, pharmacokinetics, clinical activity, and immunological parameters. The first patient treated with each dose level was observed for 16 days (48 h after the second dose) for the occurrence of dose-limiting toxicity before treatment of the second patient. The second and third patients were treated at least 48 h apart. If no dose-limiting toxicity was observed, additional patients were enrolled at the same dose level without sequential dosing. Patients were followed for adverse events for a 3-week dose-limiting toxicity evaluation period. If no patient of three (dose levels 1 and 2) or no more than one of six patients (dose level 3) had a dose-limiting toxicity 3 weeks after initial treatment, the dose level was determined to have an acceptable toxicity profile to allow escalation to the next dose level. Patients who required discontinuation of avelumab before the end of the dose-limiting toxicity evaluation period for reasons other than the occurrence of a dose-limiting toxicity could be replaced.
Avelumab was supplied as a 10 mg/mL solution. Avelumab was given intravenously as a 1-h infusion every 2 weeks. The first two doses were given in the inpatient setting to monitor safety and do pharmacokinetics and correlative blood draws. Safety was assessed continuously with weekly visits during the first 4 weeks and then biweekly thereafter, and included assessment of performance status and medical history; physical examination and clinical laboratory tests (haematology, serum chemistry, and hepatic panels) were also done. Additional laboratory assessments included a complete urinalysis and an ECG every 2 weeks through to 13 weeks and then every 4 weeks thereafter. Laboratory tests of endocrine function (thyroid, pituitary, and adrenal glands) were done at 6-week intervals. Adverse events and laboratory abnormalities were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. A dose-limiting toxicity was defined as a grade 3 or worse adverse event occurring within the dose- limiting toxicity evaluation period of the 3 + 3 dose-escalation cohorts and confirmed by the study’s safety monitoring committee as related or relevant to treatment with avelumab. Exceptions were grade 3 infusion-related reaction resolving within 6 h and controlled with medical management; transient (≤6 h) grade 3 influenza-like symptoms or pyrexia controlled with medical management; grade 3 fatigue, local reaction, headache, nausea, or emesis that resolved to a maximum severity of grade 1 within 24 h; diarrhoea, skin toxicity, or increased liver enzyme (alanine aminotransferase, aspartate aminotransferase, or γ glutamyltransferase) that resolved to a maximum severity of grade 1 within 7 days after initiation of medical management (eg, immunosuppressant treatment). Assessment of adverse events was continued with a clinical visit and safety assessment 7–28 days after completion of treatment and telephone assessment at 3-month intervals thereafter. Infusion-related reaction was an adverse event of special interest and was assessed by investigators according to the occurrence of fever, chills, or rigors occurring on the day of infusion (post dosing) or the following day. After observations of infusion-related reactions, mandatory premedication was implemented from Jan 29, 2014. Premedication was an antihistamine (eg, 25–50 mg diphenhydramine) and paracetamol (acetaminophen; eg, 500–650 mg intravenous or oral equivalent) given 30–60 min before each dose of avelumab. The protocol amendment was approved by the principal and coordinating investigator of the trial (JLG), the National Cancer Institute’s Institutional Review Board, individuals employed by the sponsor with responsibility for the trial, and relevant regulatory authorities.
Tumours were assessed every 6 weeks based on RECIST version 1.1 using contrast-enhanced CT scans when possible. If clinically appropriate (eg, for tumours in specific body sites), MRI, PET, bone scan, or other imaging was also done. If a patient completed therapy without disease progression, radiographic assessments continued until disease progression.
The trial duration for each patient was up to about 30 weeks, including an 18-day screening period, followed by treatment until confirmed progression, unacceptable toxicity, or any other criterion for withdrawal occurred, and finally an end-of-treatment visit 4 weeks after the final dose of avelumab was given. Treatment was permanently discontinued if any grade 3 or worse adverse event occurred (with the exception of transient [≤6 h] influenza-like symptoms or pyrexia controlled with medical management; fatigue, local infusion-related reaction, headache, nausea, or emesis that resolved to grade ≤1 within 24 h; single laboratory values outside of the normal range that were unrelated to study treatment and without clinical correlate [except for elevation in liver enzyme concentrations] that resolved to grade ≤1 within 7 days; and tumour flare, defined as local pain, irritation, or rash localised at sites of known or suspected malignant tissue) or recurring grade 2 treatment-related adverse events. Grade 2 adverse drug reactions were managed by dose modifications (changes in the infusion rate) and dose delays, and those that did not resolve to grade 1 or less by the end of the next cycle led to permanent discontinuation of avelumab. Preplanned dose level reductions were not allowed; however, interruptions in delivering the planned dose that resulted in an actual dose that was less than 90% of the planned dose were defined as dose reductions.
Samples were drawn for pharmacokinetic analysis prior to initial dosing and then on days 1, 2, 3, 8, 15, 29, 43, and 85 and every 6 weeks through to week 25. Pharmacokinetic and dose proportionality of avelumab were characterised by standard non-compartmental analysis based on rich serum concentration-time data obtained over the 2-week dosing interval. Pharmacokinetic parameters were area under the curve (AUC) from the time of dosing to the time of last observation (AUC0-t), during the first dose interval (AUC0–336), or extrapolated to infinity (AUC0−∞), calculated by linear trapezoidal summation; the maximum serum concentration (Cmax); and elimination half-life (t1/2), determined as 0·693 divided by the terminal elimination rate constant (λz).
Serum samples for assessing cytokine concentrations were obtained on day 1 (baseline), and on days 3 (48 h plus or minus 6 h), 8, 15, 29, 43, 45, 50, and 85. Additional methods for assessment of cytokine release by PBMCs are provided in the appendix (p 2). Serum concentrations of avelumab were measured by a three-step immunoassay in the Gyrolab platform (Gyros, Warren, NJ, USA) using streptavidin-coated beads, biotinylated recombinant human B7-H1/PD-L1–Fc chimera as capture reagent, and an Alexa Fluor-647–labelled recombinant human B7-H1/PD-L1–Fc chimera as detection reagent.
Samples for monitoring development of human antidrug antibodies were drawn before initial dosing on days 15, 29, 43, and 85, and every 6 weeks through to week 25. Antidrug antibodies to avelumab were detected with an electrochemiluminescent immunoassay using Meso Scale Discovery technology (Rockville, MD, USA), validated according to current US FDA and European Medicines Agency guidelines for immunogenicity testing, including those for specificity. Antidrug antibody detection was based on binding to labelled avelumab. Briefly, serum samples were incubated in an acidic solution to dissociate existing immune complexes. During neutralisation, excess biotinylated avelumab was introduced to capture anti-avelumab antibodies, and complexes were captured on streptavidin-coated microplates. Quantification involved addition of ruthenylated avelumab and measurement of light emission.
To assay PD-L1 target occupancy by avelumab, PBMCs were isolated from patients at baseline and after one treatment cycle (on day 15 before treatment) and immediately frozen. After thawing to 4°C, non-specific binding was saturated by adding human IgG (anti-HEL) and incubating for 15 min. Next, either a biotinylated version of avelumab or an inactive biotinylated control (MSB0010707C) was added to duplicate wells, followed by incubation on ice for 45 min. Binding was detected with streptavidin APC. Target occupancy of PD-L1 on CD3+ T cells was determined with flow cytometry based on the difference in mean fluorescence intensity between baseline and post-treatment samples.
As part of the pharmacodynamic profile analyses, immune response samples, including PBMCs for analysis of immune cell subsets and serum for cytokine profile and soluble immune factors (including interferon γ, interleukin 6, and tumour necrosis factor a; for a full list of cytokines and soluble factors analysed see appendix p 2) were drawn on days 1, 3, 8, 15, 43, and 85 and every 6 weeks up to week 25. Archived tumour tissue was also collected at baseline. Multicolour flow cytometry was done on frozen PBMC samples to identify CD4+ and CD8+ T cells, B cells, regulatory T cells (Tregs), NK cells, NK T cells, conventional dendritic cells (cDCs), plasmacytoid DCs (pDCs), and myeloid derived suppressor cells (MDSCs), as well as PD-L1 expression within each of these subsets, as previously described.16 Briefly, one vial of PBMCs containing 107 cells was thawed per patient before therapy and following one cycle (at around day 15), three cycles (at around day 43), and nine cycles (at around day 127) of avelumab, and was stained with multiple antibody panels and acquired on a BD LSRII cytometer. Data were analysed using FlowJo with non-viable cells excluded and negative gates set based on fluorescence minus one controls. The frequency of all subsets was calculated as a percentage of PBMCs to help to eliminate the bias that could occur in the smaller populations with fluctuations in parental leukocyte subpopulations.
Outcomes
The primary endpoint of the dose-escalation part of the study was the safety of avelumab in ascending-dose cohorts by investigator assessment, defined by the occurrence of dose-limiting toxicities during the evaluation period and identification of the maximum tolerated dose (the highest dose at which fewer than two of six patients experience a dose-limiting toxicity). Secondary endpoints in the dose-escalation part of the study were the number, severity, and duration of treatment-emergent adverse events and treatment-related adverse events according to NCI-CTCAE version 4.0 (treatment-emergent adverse events were defined as adverse events with onset dates during the on-treatment period, or which worsened during the on-treatment period and treatment-related adverse events were defined as treatment-emergent adverse events considered by the investigator to be possibly related to avelumab treatment); pharmacokinetic and pharmacodynamic profiles (immunological effects); best overall response by RECIST version 1.1; and assessment of antidrug antibody formation.
Statistical analysis
The planned number of evaluable patients during the dose-escalation part of the trial (the dose-limiting toxicity population) was at least 18 and up to 66 patients, derived from the 3 + 3 study design. The safety analysis population for the dose-escalation part was defined as all patients who received at least one dose of avelumab. The planned cutoff date for analysis was the timepoint at which all patients had completed at least three 2-week treatment cycles (ie, at least 6 weeks after the last patient had received a first dose of avelumab). For the determination of the maximum tolerated dose, individual patient data were reported and in the final analysis of the dose-limiting toxicity population, the number and proportion of patients who experienced a dose-limiting toxicity or treatment-emergent adverse event during the dose-limiting toxicity evaluation period were analysed. Adverse events were reported and analysed for all patients in the dose-escalation safety population. Analysis of pharmacokinetic variables was done on the pharmacokinetic population comprised of all patients from the dose-escalation part plus patients with rich pharmacokinetic sampling from selected expansion cohorts. Patients with rich pharmacokinetic sampling were enrolled within two disease-specific expansion cohorts at the same study site, and had serum samples obtained at multiple early timepoints to increase the robustness of the pharmacokinetic data available. Pharmacokinetic parameters were estimated with the validated software Phoenix/WinNonlin 6.3. All other statistical analyses were done with SAS version 9.1.3 or higher, or R, version 2.10.1 or higher.
Results
Between Jan 31, 2013, and Oct 8, 2014, 54 patients were assessed and 53 eligible patients were enrolled at the Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA (figure 1). Median age at the time of enrolment was 58 years (IQR 49–64) and patients had a range of solid tumours (table 1). At data cutoff on Nov 20, 2015, 53 patients had received at least one dose of avelumab and all were evaluable for the safety analysis. For dose level 1 we enrolled four patients, for dose level 2 we enrolled 13 patients, for dose level 3 we enrolled 15 patients, and for dose level 4 we enrolled 21. One patient receiving dose level 1 had to be replaced because of rapid disease progression and death before completion of the dose-limiting toxicity evaluation period. The dose-limiting toxicity analysis set comprised 18 patients: three at dose level 1 (1 mg/kg), three at dose level 2 (3 mg/kg), six at dose level 3 (10 mg/kg), and six at dose level 4 (20 mg/kg).
Figure 1: Trial profile.
Table 1:
Patient characteristics
| 1 mg/kg (n=4) | 3 mg/kg (n=13) | 10 mg/kg (n=15) | 20 mg/kg (n=21) | |
|---|---|---|---|---|
| Age (years) | 60.5 (56.0–64.5) | 59.0 (50.0–67.0) | 56.0 (49.0–66.0) | 59.0 (46.0–61.0) |
| Age group | ||||
| <65 years | 3 (75%) | 9 (69%) | 10 (67%) | 18 (86%) |
| ≥65 years | 1 (25%) | 4 (31%) | 5 (33%) | 3 (14%) |
| ECOG performance status | ||||
| 0 | 1 (25%) | 4 (31%) | 8 (53%) | 9 (43%) |
| 1 | 3 (75%) | 9 (69%) | 7 (47%) | 12 (57%) |
| Sex | ||||
| Men | 2 (50%) | 4 (31%) | 7 (47%) | 14 (67%) |
| Women | 2 (50%) | 9 (69%) | 8 (53%) | 7 (33%) |
| Race | ||||
| White | 3 (75%) | 13 (100%) | 11 (73%) | 20 (95%) |
| Black or African American | 0 | 0 | 2 (13%) | 0 |
| Asian | 1 (25%) | 0 | 2 (13%) | 0 |
| Other | 0 | 0 | 0 | 1 (5%) |
| Primary tumour | ||||
| Adrenocortical | 0 | 0 | 0 | 2 (10%) |
| Anal (squamous) | 0 | 2 (15%) | 2 (13%) | 0 |
| Bladder | 0 | 0 | 0 | 2 (10%) |
| Breast | 0 | 1 (8%) | 1 (7%) | 2 (10%) |
| Cervical | 0 | 0 | 0 | 1 (5%) |
| Cholangiocarcinoma | 1 (25%) | 0 | 0 | 0 |
| Chordoma | 0 | 1 (8%) | 0 | 0 |
| Colorectal | 0 | 0 | 2 (13%) | 0 |
| Medullary thyroid | 0 | 0 | 0 | 1 (5%) |
| Mesothelioma | 0 | 2 (15%) | 0 | 1 (5%) |
| Nasopharyngeal | 0 | 0 | 1 (7%) | 0 |
| Non-small-cell lung cancer | 0 | 1 (8%) | 1 (7%) | 2 (10%) |
| Oesophagus (squamous) | 0 | 0 | 0 | 1 (5%) |
| Ovarian | 0 | 1 (8%) | 0 | 0 |
| Pancreatic | 2 (50%) | 2 (15%) | 2 (13%) | 1 (5%) |
| Renal cell | 1 (25%) | 1 (8%) | 1 (7%) | 4 (19%) |
| Synovial sarcoma | 0 | 0 | 0 | 1 (5%) |
| Thymic epithelial tumours | 0 | 0 | 5 (33%) | 3 (14%) |
| Tongue (spindle) | 0 | 1 (8%) | 0 | 0 |
| Trachea (squamous) | 0 | 1 (8%) | 0 | 0 |
| Previous lines of anticancer therapy | ||||
| 1 | 1 (25%) | 4 (31%) | 1 (7%) | 2 (10%) |
| 2 | 2 (50%) | 3 (23%) | 1 (7%) | 6 (29%) |
| 3 | 1 (25%) | 2 (15%) | 2 (13%) | 4 (19%) |
| ≥4 | 0 | 4 (31%) | 11 (73%) | 9 (43%) |
Data are median (IQR) or n (%). ECOG=Eastern Cooperative Oncology Group.
Median treatment duration was 7·1 weeks (IQR 3·1–10·9) for dose level 1, 12·0 weeks (6·0–17·9) for dose level 2, 12·0 weeks (4·3–24·0) for dose level 3, 12·1 weeks (6·0–17·7) for dose level 4, and 12·0 weeks (5·9–17·9) for all cohorts combined. Median number of treatment administrations was 3·5 (IQR 1·5–5·5) for dose level 1, 6·0 (3·0–9·0) for dose level 2, 6·0 (2·0–12·0) for dose level 3, 6·0 (3·0–9·0) for dose level 4, and 6·0 (3·0–9·0) for all cohorts combined. No preplanned dose reductions were allowed as per the protocol; however, dose administration was interrupted in one patient at dose level 4 because of a treatment-related adverse event (grade 3 increased blood creatine phosphokinase), resulting in administration of a dose that was less than 90% of the planned dose. Dose delays occurred in no patients at dose level 1, four patients at dose level 2, six patients at dose level 3, and four patients at dose level 4. Median duration of follow-up for all 53 patients was 22·2 months (IQR 19·7–26·3).
Of 18 patients treated in the dose-limiting toxicity analysis set, no dose-limiting toxicities were noted at dose levels 1, 2, or 3, and one dose-limiting toxicity was reported at dose level 4 in a patient with metastatic thymoma. The array of symptoms that this patient had irrespective of treatment included myositis, neurological dysfunction, and transaminitis, as previously described17–20 (classified as autoimmune disorder in table 2), and increased blood creatine phosphokinase. All dose levels had fewer than two dose-limiting toxicities; thus, the maximum tolerated dose was not reached.
Table 2:
Treatment-related adverse events occurring at any grade in ≥10% of patients in any cohort or at grade ≥3 in any patient
| 1 mg/kg dose (n=4) |
3 mg/kg dose (n=13) |
10 mg/kg dose (n=15) |
20 mg/kg dose (n=21) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Fatigue | 0 | 0 | 0 | 5 (39%) | 0 | 0 | 10 (67%) | 1 (7%) | 0 | 5 (24%) | 0 | 0 |
| Influenza-like illness | 1 (25%) | 0 | 0 | 1 (8%) | 0 | 0 | 3 (20%) | 0 | 0 | 6 (29%) | 0 | 0 |
| Pyrexia | 2 (50%) | 0 | 0 | 2 (15%) | 0 | 0 | 4 (27%) | 0 | 0 | 0 | 0 | 0 |
| Chills | 2 (50%) | 0 | 0 | 0 | 0 | 0 | 2 (13%) | 0 | 0 | 2 (10%) | 0 | 0 |
| Allergic rhinitis | 0 | 0 | 0 | 2 (15%) | 0 | 0 | 1 (7%) | 0 | 0 | 1 (5%) | 0 | 0 |
| Increased blood creatine phosphokinase | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 1 (5%) | 1 (5%) | 1 (5%) |
| Diarrhoea | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 2 (13%) | 0 | 0 | 1 (5%) | 0 | 0 |
| Infusion-related reaction | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 3 (14%) | 0 | 0 |
| Lymphopenia | 0 | 0 | 0 | 2 (15%) | 0 | 0 | 2 (13%) | 0 | 0 | 0 | 0 | 0 |
| Decreased lymphocyte count | 0 | 0 | 0 | 2 (15%) | 0 | 0 | 1 (7%) | 1 (7%) | 0 | 0 | 0 | 0 |
| Myalgia | 1 (25%) | 0 | 0 | 1 (8%) | 0 | 0 | 0 | 0 | 0 | 2 (10%) | 0 | 0 |
| Increased aspartate aminotransferase | 1 (25%) | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 0 | 0 |
| Autoimmune disorder | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 1 (7%) | 0 | 1 (5%) | 0 |
| Hyperglycaemia | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 2 (13%) | 0 | 0 | 0 | 0 | 0 |
| Increased lipase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (10%) | 1 (5%) | 0 |
| Nausea | 0 | 0 | 0 | 0 | 0 | 0 | 2 (13%) | 0 | 0 | 1 (5%) | 0 | 0 |
| Rash | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 1 (5%) | 0 | 0 |
| Increased alanine aminotransferase | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 0 | 0 |
| Increased amylase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 1 (5%) | 0 |
| Hypotension | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (10%) | 0 | 0 |
| Malaise | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 0 | 0 |
| Increased blood alkaline phosphatase | 0 | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lower abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 0 | 0 |
| Hypocalcaemia | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Insomnia | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Data are n (%). No grade 5 treatment-related adverse events occurred.
Table 2 summarises treatment-related adverse events in the safety analysis set of 53 patients and table 3 presents an overview of the safety profile for avelumab. Across all dose levels, common treatment-related adverse events (occurring in ≥10% of patients) of any grade included fatigue (21 [40%] of 53 patients), influenza-like symptoms (11 [21%]), fever (eight [15%]), and chills (six [11%]; table 2). Grade 3–4 treatment-related adverse events occurred in nine (17%) of 53 patients, with autoimmune disorder (n=3), increased blood creatine phosphokinase (n=2), and increased aspartate aminotransferase (n=2) each occurring in more than one patient (autoimmune disorder in two patients at 10 mg/kg and one patient at 20 mg/kg, increased blood creatine phosphokinase in two patients at 20 mg/kg, and increased aspartate aminotransferase in one patient at 1 mg/kg and one patient at 10 mg/kg). Potential immune-related adverse events related to avelumab were hypothyroidism (one [8%] of 13 at dose level 2), autoimmune disorder (two [13%] of 15 at dose level 3 and one [5%] of 21 at dose level 4), and myositis (one [5%] of 21 at dose level 4). Three patients with thymoma experienced the above noted array of autoimmune toxicities associated with antitumour effects, summarised as autoimmune disorder grade 3 or worse in table 2. Serious adverse events regardless of relation to study treatment occurred in 22 (42%) of 53 patients. Serious adverse events related to avelumab were autoimmune disorder, lower abdominal pain, fatigue, and influenza-like illness in three (20%) of 15 patients treated at dose level 3, (lower abdominal pain, fatigue, and influenza-like illness occurred in the same patient; autoimmune disorder occurred in two patients) and autoimmune disorder, increased amylase, myositis, and dysphonia in three (14%) of 21 patients (myositis and dysphonia occurred in the same patient) treated at dose level 4. Treatment-related adverse events leading to permanent discontinuation of treatment were elevated aspartate aminotransferase in one (25%) of four patients at dose level 1, autoimmune disorder in one (7%) of 15 at dose level 3, and increased creatine phosphokinase (two patients [10%]), increased amylase (one patient [5%]), autoimmune disorder (one patient [5%]), infusion-related reaction (one patient [5%]), myositis (one patient [5%]), pain in extremity (one patient [5%]), and dysphonia (one patient [5%]) in four (19%) of 21 patients treated at dose level 4. In the 20 mg/kg dose level, two patients discontinued after experiencing more than one treatment-related adverse event (one patient with increased creatinine phosphokinase and autoimmune disorder, and another patient with increased creatinine phosphokinase, myositis, pain in extremity, and dysphonia).
Table 3:
Overview of safety
| 1 mg/kg (n=4) | 3 mg/kg (n=13) | 10 mg/kg (n=15) | 20 mg/kg (n=21) | |
|---|---|---|---|---|
| Any treatment-related adverse event | 3 (75%) | 9 (69%) | 14 (93%) | 17 (21%) |
| Grade ≥3 treatment-related adverse event | 1 (25%) | 0 | 5 (33%) | 3 (14%) |
| Treatment-related adverse event leading to treatment discontinuation | 1 (25%) | 0 | 1 (7%) | 4 (19%) |
| Serious treatment-related adverse event | 0 | 0 | 3 (20%) | 3 (14%) |
| Immune-related treatment-related adverse event | 0 | 1 (8%) | 2 (13%) | 2 (10%) |
Data are n (%).
31 (58%) of 53 patients in the overall safety population died, and of these, 14 (26%) of the 31 deaths were due to disease progression. Adverse events leading to death and not related to study treatment occurred in four (8%) of 53 patients: two (50%) of four patients at dose level 1 (dyspnoea, hypoxia, and pneumonia in one patient and ascites and duodenal obstruction in one patient), none of 13 patients at dose level 2, one (7%) of 15 patients at dose level 3 (decreased appetite and disease progression), and one (5%) of 21 patients at dose level 4 (respiratory failure); cause of death was not reported or not available for the other 13 patients. No treatment-related adverse event led to death.
Infusion was stopped in the event of infusion-related reactions. If not given as premedication, acetaminophen, diphenhydramine, and ranitidine were given. In the case of rigors, meperidine was given. In all cases, patients with infusion-related reactions responded rapidly to cessation of infusion and to the described interventions. One patient in dose level 3 was given steroids. After symptoms resolved, infusion was restarted at 50% of the rate of initial infusion. In only one patient on dose level 4, an infusion-related reaction occurred after the infusion was restarted at the 50% rate, and treatment was permanently discontinued. Initially, premedication was not required, but the requirement was added after infusion-related reactions were observed. 24 (45%) of 53 patients received premedication before every dose (one [25%] of four patients at dose level 1, one [8%] of 13 at dose level 2, eight [53%] of 15 at dose level 3, and 14 [67%] of 21 at dose level 4). Of these patients, two (8% overall, 14% of the premedicated dose level 4 cohort) had an infusion-related reaction. 29 (55%) of 53 patients received at least one dose without premedication, of whom two (7%) had infusion-related reactions: one at dose level 3 and one at dose level 4. These patients were premedicated before receiving subsequent doses; one had a subsequent infusion-related reaction and was withdrawn from the study.
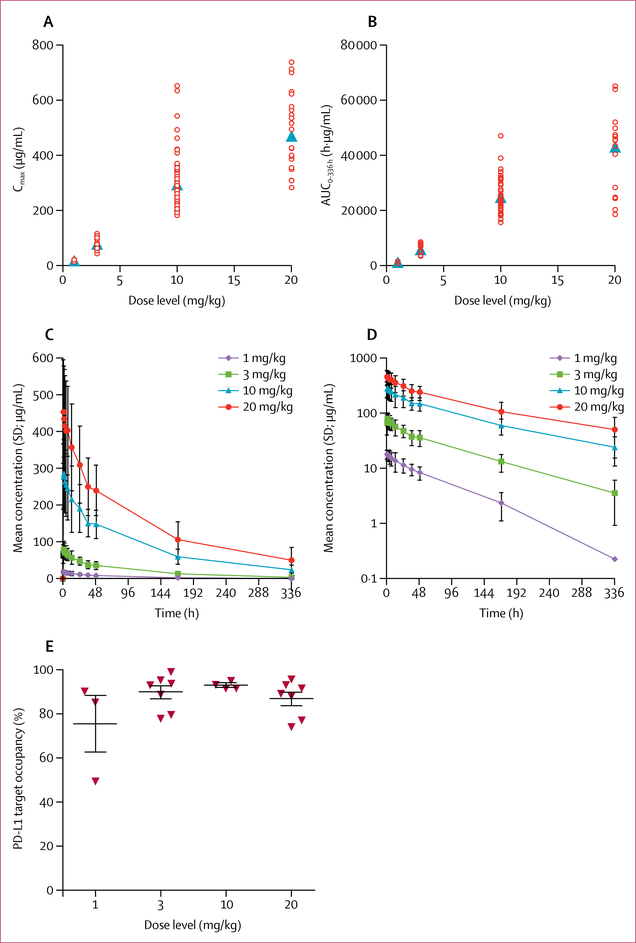
The pharmacokinetic analysis population (n=86) included all 53 patients from the phase 1a dose-escalation cohort plus 33 patients with rich pharmacokinetic sampling enrolled from the same institution within the dose-expansion part of the trial, which comprises parallel expansion in multiple solid tumour cohorts. These 33 patients had either castration-resistant prostate cancer or colorectal cancer. The avelumab dose was 1 mg/kg in four patients, 3 mg/kg in 13 patients, 10 mg/kg in 48 patients, and 20 mg/kg in 21 patients. Avelumab showed a dose-proportional increase for AUC and maximum serum concentration between doses of 3 mg/kg and 20 mg/kg (figure 2A, B). For all doses, the mean time to maximum concentration was within 1 h from the end of infusion (figure 2C, D). The geometric mean half-life for avelumab after the 1 mg/kg dose was 59·5 h (2·5 days), after the 3 mg/kg dose was 81·2 h (3·4 days), after the 10 mg/kg dose was 94·6 h (3·9 days), and after the 20 mg/kg dose was 99·1 h (4·1 days; table 4). Trough levels below 1 μg/mL were observed in some patients receiving 3 mg/kg dosing. Profiles with more than 20% AUC extrapolation beyond 336 h were excluded from AUC0−∞ and half-life calculations, which introduced a downward bias for the 10 mg/kg and 20 mg/kg doses. The similarity in half-life between these two higher dose levels suggests that target-mediated clearance was saturated at these doses. Accumulation on repeated dosing, based on Ctrough measurements over time, was slightly higher than expected from the observed half-life.
Figure 2: Pharmacokinetic and pharmacodynamic profile of avelumab after the first 1-h infusion.
(A) Relation between each avelumab dose and its maximum serum concentration (Cmax). (B) Relation between each avelumab dose and the area under the concentration-time curve (AUC) during the first dose interval (AUC0–336). Open circles represent individual patients; filled triangles represent the geometric mean. (C) Mean serum concentration of avelumab over time after treatment with different doses using a linear scale. (D) Mean serum concentration of avelumab over time after treatment with different doses using a semilog scale. (E) Target occupancy of avelumab for PD-L1 on CD3+T cells on day 15 (end of first dosing cycle) after treatment at the four different dose levels in 21 patients (1 mg/kg, n=3; 3 mg/kg, n=7; 10 mg/kg, n=4; and 20 mg/kg, n=7).
Table 4:
Summary of pharmacokinetic parameters for avelumab dose groups
| 1 mg/kg dose | 3 mg/kg dose | 10 mg/kg dose | 20 mg/kg dose | |
|---|---|---|---|---|
| Cmax (μg/mL) | ||||
| Patients, n | 4 | 13 | 48 | 21 |
| Mean (SD) | 18.7 (3.96) | 81.9 (22.1) | 310 (109) | 489 (140) |
| Geometric mean (CV%) | 18.4 (21.8%) | 78.9 (29.5%) | 294 (32.5%) | 470 (29.9%) |
| Ctrough (μg/mL) | ||||
| Patients, n | 3 | 13 | 39 | 19 |
| Mean (SD) | 0.23 (0.39) | 3.55 (2.61) | 24.0 (13.3) | 50.1 (34.6) |
| Geometric mean (CV%) | NC | NC | 20.9 (57.4%) | 36.9 (112.0%) |
| AUC0–t (h·μg/mL) | ||||
| Patients, n | 4 | 13 | 48 | 21 |
| Mean (SD) | 1040 (443) | 6080 (1970) | 24 800(9150) | 45 100 (15 600) |
| Geometric mean (CV%) | 976 (40.7%) | 5740 (37.6%) | 22 800 (48.2%) | 42 200 (40.8%) |
| AUC0–336 h (h·μg/mL) | ||||
| Patients, n | 2 | 12 | 39 | 15 |
| Mean (SD) | 1250 (591) | 6340 (1800) | 25 900 (6680) | 41 100 (14 800) |
| Geometric mean (CV%) | 1180 (52.2%) | 6080 (32.1%) | 25 200 (25.3%) | 38 300 (42.0%) |
| AUC0–∞ (h·μg/mL) | ||||
| Patients, n | 2 | 12 | 39 | 15 |
| Mean (SD) | 1290(650) | 6850 (2100) | 28 600 (7700) | 46 600 (18 500) |
| Geometric mean (CV%) | 1200 (56.7%) | 6520 (34.8%) | 27 700 (27.0%) | 42 700 (47.6%) |
| t1/2 (h) | ||||
| Patients, n | 2 | 12 | 39 | 15 |
| Mean (SD) | 61.4 (21.7) | 84.3 (21.8) | 96.6 (18.6) | 103 (27.4) |
| Geometric mean (CV%) | 59.5 (37.4%) | 81.2 (30.3%) | 94.6 (22.0%) | 99.1 (29.9%) |
Cmax=maximum serum concentration observed post dose. CV%=coefficient variation for geometric mean. Ctrough=median serum concentration at 336 h. NC=non-calculable. AUC0–t=area under the concentration-time curve from the time of dosing to the time of the last observation. AUC0–336=area under the serum concentration-time curve during the first dose interval. AUC0–∞=area under the concentration-time curve from the time of dosing extrapolated to infinity. t1/2=elimination half-life.
Antidrug antibodies were detected in two (4%) of 53 patients in the dose-escalation part of the study (both at dose level 2). One patient tested positive for antidrug antibodies at several timepoints; the study treatment was not changed, and the patient received nine doses in total until disease progression. The other patient tested positive for antidrug antibodies at a single assessment only.
As part of the pharmacodynamic profiling, minor increases in serum concentrations of tumour necrosis factor α, interferon γ, and interleukin 6 were noted in some patients at 48 h after the end of infusion, reaching two to three times the concentrations recorded at baseline; data for individual patients who received avelumab 10 mg/kg or 20 mg/kg are shown in the appendix (pp 2–3). Cytokine concentrations varied with dose and time but no clear pattern emerged. Appendix pp 4–9 show serum concentrations of interferon γ, interleukin 6, and tumour necrosis factor α for patients in the avelumab 10 mg/kg and 20 mg/kg dose groups.
In other pharmacodynamics analyses, avelumab target occupancy of PD-L1 on CD3+ T cells was assessed in 21 patients at the end of cycle 1 (day 15) before the second dose. Mean PD-L1 target occupancy (figure 2E) was 76% with 1 mg/kg (n=3), 90% with 3 mg/kg (n=7), 93% with 10 mg/kg (n=4), and 87% with 20 mg/kg (n=7).
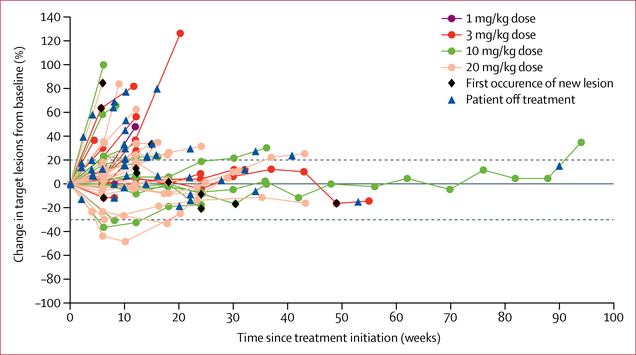
Despite the heterogeneous and heavily pretreated patient population (table 1), some evidence of clinical activity was recorded (figure 3; appendix p 10). Four (8%) of 53 patients had a partial response (two at dose level 3, one confirmed; two at dose level 4, one confirmed per standard RECIST version 1.1 definition [ie, response criteria must be met again in an assessment performed 4 weeks after initial documentation]), and 30 (57%) of 53 additional patients had stable disease, which was durable in three patients with duration of 40 weeks or more (figure 3). Three of the partial responses occurred in eight patients enrolled with thymic epithelial malignancies (one with thymic carcinoma and seven with thymoma); all three of these responders also had a complicated range of autoimmune effects, which seemed to be specific to this cohort of patients, as previously described in thymoma.16–19 The complexities of this set of patients will be discussed in a separate report. The other partial response occurred in a patient with adrenocortical carcinoma at dose level 4.
Figure 3: Clinical activity.
Plot of the percentage change in tumour measurements over time in 47 evaluable patients enrolled at dose levels 1, 2, 3, and 4 who had at least one post-baseline lesion assessment.
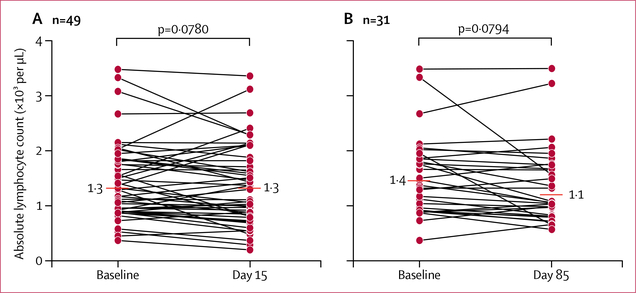
As a further part of the pharmacodynamic profiling, frozen PBMCs from 28 patients with sufficient PBMC sampling were assessed before treatment versus after one dose (day 15, n=19), three doses (day 43, n=14), and nine doses (day 127, n=16) of avelumab. We tested 21 of the first 23 patients who enrolled in the study; two patients were excluded because the patient remained on study for only 2 days or they had insufficient PBMCs at the post-treatment timepoints. The other seven of the 28 patients were selected with the purpose of assessing the potential long-term effects of avelumab on the peripheral immunome. These seven patients had received more than nine cycles of avelumab (two at dose level 2, one at dose level 3, four at dose level 4) and had sufficient PBMCs. The 28 patients examined provided a valid representation of the dose levels of avelumab tested in the study (two given 1 mg/kg; nine given 3 mg/kg; eight given 10 mg/kg; and nine given 20 mg/kg). Stable disease occurred in 23 (82%) of 28 patients, including confirmed stable disease in two patients at dose level 1, seven at dose level 2, six at dose level 3, and eight at dose level 4; unconfirmed partial response occurred in one patient at dose level 4. No significant change in absolute lymphocyte count was noted from baseline to day 15 (figure 4A) or day 85 (figure 4B) of avelumab treatment. Furthermore, no changes were noted in the nine standard immune cell subsets (CD4+ T cells, CD8+ T cells, NK cells, Tregs cells, MDSCs, NK T cells, pDCs, cDCs, and B cells) or in the frequency of PD-L1+ cells within these standard subsets after one, three, or nine cycles of avelumab (appendix p 11).
Figure 4: Median absolute lymphocyte count pre-treatment to post treatment.
Absolute lymphocyte count at (A) baseline and day 15 (n=49) and (B) baseline and day 85 (n=31).
Discussion
This dose-escalation study of avelumab in patients with advanced solid tumours assessed 18 patients during the dose-limiting toxicity evaluation period and 53 patients overall at four dose levels. One patient had dose-limiting toxicity (autoimmune disorder and increased creatine phosphokinase) at the highest dose level assessed (20 mg/kg), and the maximum tolerated dose was not reached. Based on pharmacokinetics, target occupancy, and immunological analysis, we chose 10 mg/kg given every 2 weeks as the dose for ongoing development. Although avelumab mediates ADCC cytotoxicity in vitro, we found no evidence of targeting peripheral immune cells expressing PD-L1. Evidence of clinical activity was noted in this heavily pretreated and heterogeneous patient population.
Although in-vivo murine experiments have shown that an IgG1 capable of mediating ADCC, while possibly detrimental in PD-1-targeting antibodies, increases the antitumour efficacy of PD-L1-targeting antibodies,21 avelumab did not have a substantial effect on absolute lymphocyte count or the number of circulating PD-L1-expressing immune cells.16 These data greatly allay concerns that avelumab—an IgG1 anti-PD-L1 antibody capable of mediating ADCC—might deplete tumour-specific effector cells expressing PD-L1. These data also support our previous finding that avelumab does not induce lysis of PBMCs when cocultured with purified autologous NK cells in vitro.10 In a subsequent study, we also showed that the addition of avelumab in an in-vitro stimulation assay resulted in greater antigen-specific immune activation than without avelumab, indicating that it does not lyse cells important for immune stimulation.21 However, so far, no data have shown that ADCC contributes to the clinical activity of avelumab.
At all dose levels, patients were assessed for safety and clinical activity at each visit, resulting in assessment of dose-limiting toxicities during the first 3 weeks of treatment and longer-term assessment of safety through extended and regular follow-up. The safety profile of avelumab noted in the dose-escalation part of this trial is similar to preliminary pooled data from expansion cohorts enrolled in this trial22 and to data for other anti-PD-L1 and anti-PD-1 antibodies,8,23,24 apart from the slightly increased rate of infusion-related reactions, which occurred mainly after the first infusion. Infusion-related reactions, such as influenza-like symptoms, are often associated with release of proinflammatory cytokines by direct activation of various immunocompetent cells.25 Although our in-vitro studies with human PBMCs indicated the potential for avelumab to induce cytokine release after 6 h and 24 h of incubation, concentrations of proinflammatory cytokines in serum measured at 48 h after the end of infusion showed only minor increases. Because cytokine release has been reported to occur during and shortly after therapeutic antibody infusion,26,27 cytokine concentrations noted at 48 h might be indicative of increased concentrations shortly after the infusion. We currently do not have adequate data to analyse the relation between cytokine concentrations immediately after avelumab infusions and specific toxicities; this association will be investigated further in ongoing clinical studies. Infusion-related reactions were manageable in all patients enrolled in the dose-escalation part of the trial and were resolved quickly with standard agents such as antihistamines, meperidine, acetaminophen, and, rarely, corticosteroids. Consistent with standard clinical practice, patients who had an infusion-related reaction had their infusion restarted at 50% reduced rate, and only one of four patients had a recurrent event, suggesting that reducing the rate might help to avoid recurrence. This aspect of avelumab requires additional caution, but it is manageable with techniques similar to those used with other monoclonal antibodies currently used in standard clinical practice.
Evidence of clinical activity was noted in patients treated with avelumab in the dose-escalation part of this phase 1a trial. Of four partial responses, three occurred in patients with thymic tumours, and response in these patients was associated with autoimmune effects. These effects could be related to PD-L1 blockade and T-cell activation or ADCC activity, or both, by avelumab. However, autoimmune disorders are a commonly recognised feature of thymic tumours.17–20 The other partial response occurred in a patient with adrenocortical carcinoma. Additionally, stable disease was recorded in 30 of 53 patients across a heterogenous array of advanced solid tumours. Although antitumour activity was not a primary endpoint of this study, partial response and stable disease data were encouraging and supported further studies of durable clinical activity and safety in tumour-type-specific cohorts enrolled in the dose-expansion part of this trial.
The 10 mg/kg dose and 2-week dosing schedule were selected for further development based on a combination of the adequate safety and tolerability noted in this study, in addition to pharmacokinetic studies and pharmacokinetic modelling, target occupancy studies, and animal studies. The exposures associated with the 10 mg/kg dose given every 2 weeks were shown to achieve the high target occupancy of PD-L1 to enable clinical assessment of potential treatment benefit with avelumab. Although target occupancy was higher than 90% in four of seven patients tested at the 3 mg/kg dose, trough levels below 1 μg/mL were observed at several dosing occasions in some patients receiving 3 mg/kg dosing. In-vitro studies have previously shown that 1 μg/mL was the serum level required to ensure more than 90% target occupancy,28 and trough concentrations with 10 mg/kg dosing were consistently above this threshold. A limitation of our data for target occupancy is that only a subset of patients (21 of 53) had PBMC samples available for analysis because of challenges with PBMC sample preparation, storage, and cell survival. These early data do not represent a comprehensive assessment of optimal avelumab dosing, and additional questions relating to dose and schedule are planned to be addressed in further clinical studies.
The half-life of avelumab (3·9 days for the 10 mg/kg dose and 4·1 days for the 20 mg/kg dose) is relatively short compared with those of other anti-PD-1-PD-L1 monoclonal antibodies, including nivolumab (12–20 days),29 pembrolizumab (14–22 days),30 and atezolizumab31 and durvalumab24 (both about 21 days). The isoelectric point of avelumab is quite high at 8·9–9·3 (Neuteboom B, unpublished), and a high antibody isoelectric point has been found to correlate with increased tissue distribution, tumour uptake, and hepatic clearance, resulting in shorter circulating half-life.32,33 Thus, the high isoelectric point of avelumab might help to explain its relatively short half-life.
Like other agents targeting the PD-1-PD-L1 pathway, avelumab has the potential to be combined with T cell-activating agents, including therapeutic cancer vaccines and cytokines or other T-cell therapies (eg, chimeric antigen receptor T cells). However, its ability to mediate ADCC might give avelumab a unique advantage over other PD-1 or PD-L1-targeting agents in combination with agents that drive NK cell activation or expansion or with the adoptive transfer of expanded NK cells. The efficacy and safety of avelumab at a 10 mg/kg dose is being examined in several phase 3 trials, including single-agent (NCT02395172, NCT02576574, NCT02603432, NCT02625610, and NCT02625623) and combination (NCT02580058, NCT02684006, NCT02718417, and NCT02952586) studies, with durable responses already reported with single-agent avelumab in a phase 2 study in patients with metastatic Merkel cell carcinoma,9 and a phase 1b cohort with previously treated non-small-cell lung cancer.15 This phase 3 programme will provide a detailed examination of the therapeutic potential of avelumab in a range of tumor types.
Supplementary Material
Research in context.
Evidence before this study
Before we began this study, evidence of clinical activity of PD-1-PD-L1 blockade had been reported in the literature. We searched PubMed using the terms “PD-1” and “PD-L1” in June, 2016, which identified a range of literature, including various preclinical and clinical studies. Published preclinical work was supported by our own preclinical work showing the potential of inhibiting this pathway to allow activated T cells to kill tumour cells in an efficient way. Our preclinical work also showed that avelumab, an IgG1 antibody with an unmodified Fc portion, could mediate antibody-dependent cellular cytotoxicity (ADCC), making it unique compared with similar anti-PD-1 and anti-PD-L1 agents in development. In addition, avelumab has been shown to produce rapid and durable tumour responses in patients with metastatic Merkel cell carcinoma progressed after chemotherapy and is approved in the USA for this indication.
Added value of this study
Before this study, the pharmacodynamics, pharmacokinetics, safety, and immunological activity of avelumab in human beings was unknown. This phase 1a dose-escalation study aimed to establish the safety and pharmacodynamic and pharmacokinetic profiles, while showing that avelumab did not negatively affect the quantity of any immune cell subsets in peripheral blood mononuclear cells (PBMCs) or the absolute lymphocyte count, despite its potential to induce ADCC in vitro when binding to PD-L1-expressing cells.
Implications of all the available evidence
The results of this study provide a basis for further clinical investigations of avelumab as an anticancer treatment. The findings build on the existing knowledge of PD-1 or PD-L1 axis blockade by showing no negative immunological effect on PBMCs with a fully human IgG1 PD-L1-targeting antibody capable of inducing ADCC. Avelumab has a safety profile similar to that of other PD-1 or PD-L1-targeting monoclonal antibodies, but with a shorter half-life. This study provides initial evidence of the clinical activity of avelumab in various types of solid tumours.
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health, and through a Cooperative Research and Development Agreement between EMD Serono (a business of Merck KGaA, Darmstadt, Germany) and the NCI. Sponsorship from Merck KGaA, Darmstadt, Germany, was provided as part of an alliance between Merck KGaA and Pfizer, New York, NY, USA. We thank the patients involved in this study and their families. We express appreciation to the nurses, medical oncology fellows, and consultation services at the National Institutes of Health Clinical Center for their excellent patient care. We thank the study teams at Merck KGaA, Darmstadt, Germany, and EMD Serono Research & Development Institute, Billerica, MA, USA (a business of Merck KGaA, Darmstadt, Germany), including Ti Cai (EMD Serono) for support with the target occupancy studies. We thank Bonnie L Casey and Debra Weingarten for their editorial assistance in the preparation of this report and ClinicalThinking, Hamilton, NJ, USA, for assistance with data checking and manuscript formatting (funded by Merck KGaA and Pfizer).
Funding National Cancer Institute and Merck KGaA.
Role of the funding source
Funding from the National Cancer Institute’s Center for Cancer Research Intramural Research Program, and via a Cooperative Research and Development Agreement with EMD Serono Research & Development Institute, Billerica, MA, USA (a business of Merck KGaA, Darmstadt, Germany), was used toward the design and implementation of the study, data collection, data management, data analysis, data interpretation, and writing of the report. EMD Serono provided the drug and contributed to the design and implementation of the study, data collection, data management, data analysis, data interpretation, and writing of the report. All authors had full access to the data used to write the report, and the corresponding author had final responsibility for the decision to submit for publication.
Footnotes
Declaration of interests
BN, AvH, and KC are current employees of Merck KGaA (Darmstadt, Germany) or EMD Serono Research & Development Institute, (Billerica, MA, USA; a business of Merck KGaA, Darmstadt, Germany). AvH holds stock in Merck KGaA. J-MC was an employee of EMD Serono when the study was done and is currently an employee of Agenusbio and holds stock in Agenusbio. All other authors declare no competing interests.
This study is registered with ClinicalTrials.gov, number NCT01772004.
Contributor Information
Christopher R Heery, Laboratory of Tumor Immunology and Biology.
Geraldine O’Sullivan-Coyne, Genitourinary Malignancies Branch.
Ravi A Madan, Genitourinary Malignancies Branch.
Lisa Cordes, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; Pharmacy Department, National Institutes of Health Clinical Center, Bethesda, MD, USA.
Arun Rajan, Thoracic and Gastrointestinal Oncology Branch.
Myrna Rauckhorst, Office of Research Nursing.
Elizabeth Lamping, Office of Research Nursing.
Israel Oyelakin, Laboratory of Tumor Immunology and Biology.
Jennifer L Marté, Genitourinary Malignancies Branch.
Lauren M Lepone, Laboratory of Tumor Immunology and Biology.
Renee N Donahue, Laboratory of Tumor Immunology and Biology.
Italia Grenga, Laboratory of Tumor Immunology and Biology.
Jean-Marie Cuillerot, EMD Serono, Billerica, MA, USA.
Berend Neuteboom, EMD Serono, Billerica, MA, USA.
Anja von Heydebreck, Merck KGaA, Darmstadt, Germany.
Kevin Chin, EMD Serono, Billerica, MA, USA.
Jeffrey Schlom, Laboratory of Tumor Immunology and Biology.
James L Gulley, Laboratory of Tumor Immunology and Biology; Genitourinary Malignancies Branch.
References
- 1.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 2015; 125: 3384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–30. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–32. [DOI] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–28. [DOI] [PubMed] [Google Scholar]
- 7.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEyNoTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg J, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387:1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016; 17: 1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyerinas B, Jochems C, Fantini M, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015; 3: 1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna S, Thomas A, Abate-Daga D, et al. Malignant mesothelioma effusions are infiltrated by CD3+ T cells highly expressing PD-L1 and the PD-L1+ tumor cells within these effusions are susceptible to ADCC by the anti-PD-L1 antibody avelumab. J Thorac Oncol 2016; 11: 1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii R, Friedman ER, Richards J, et al. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget 2016; 7: 33498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandeveer AJ, Fallon JK, Tighe R, Sabzevari H, Schlom J, Greiner JW. Systemic immunotherapy of non-muscle invasive mouse bladder cancer with avelumab, an anti-PD-L1 immune checkpoint inhibitor. Cancer Immunol Res 2016; 4: 452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii R, Friedman ER, Richards J, et al. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget 2016; 7: 33498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017; published online March 31. 10.1016/S1470-2045(17)30240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepone LM, Donahue RN, Grenga I, et al. Analyses of 123 peripheral human immune cell subsets: Defining differences with age and between healthy donors and cancer patients not detected in analysis of standard immune cell types. J Circ Biomark 2016; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajan A, Kotlyar D, Giaccone G. Acute autoimmune hepatitis, myositis, and myasthenic crisis in a patient with thymoma. J Thorac Oncol 2013; 8: e87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue Y, True LD, Martins RG. Thymic carcinoma associated with paraneoplastic polymyositis. Proc Am Soc Clin Oncol 2009; 27: e33–34. [DOI] [PubMed] [Google Scholar]
- 19.Thomas A, Rajan A, Berman A, Giaccone G. Multiorgan autoimmune manifestations associated with thymoma. J Thorac Oncol 2015; 10: e5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly RJ, Browne SK, Rajan A, Giaccone G. Thymoma-associated paraneoplastic polymyositis. Proc Am Soc Clin Oncol 2010; 28: e378. [DOI] [PubMed] [Google Scholar]
- 21.Grenga I, Donahue RN, Lepone LM, Richards J, Schlom J. A fully human IgG1 anti-PD-L1 MAb in an in vitro assay enhances antigen-specific T-cell responses. Clin Transl Immunol 2016; 5: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly K, Patel MR, Infante JR, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic or locally advanced solid tumors: assessment of safety and tolerability in a phase I, open-label expansion study. Proc Am Soc Clin Oncol 2015; 33: [Google Scholar]
- 23.Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016; 13: 473–86. [DOI] [PubMed] [Google Scholar]
- 24.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016; 17: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Descotes J, Gouraud A. Clinical immunotoxicity of therapeutic proteins. Expert Opin Drug Metab Toxicol 2008; 4: 1537–49. [DOI] [PubMed] [Google Scholar]
- 26.Freeman CL, Morschhauser F, Sehn L, et al. Cytokine release in patients with CLL treated with obinutuzumab and possible relationship with infusion-related reactions. Blood 2015; 126: 2646–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas K, Eisele J, Rodriguez-Leal FA, Hainke U, Ziemssen T. Acute effects of alemtuzumab infusion in patients with active relapsing-remitting MS. Neurol Neuroimmunol Neuroinflam 2016; 3: e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heery CR, O’Sullivan Coyne GH, Marte JL, et al. Pharmacokinetic profile and receptor occupancy of avelumab (MSB0010718C), an anti-PD-L1 monoclonal antibody, in a phase I, open-label, dose escalation trial in patients with advanced solid tumors. J Clin Oncol 2015; 33: [Google Scholar]
- 29.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015; 21: 4286–93. [DOI] [PubMed] [Google Scholar]
- 31.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem 2010; 21: 2153–63. [DOI] [PubMed] [Google Scholar]
- 33.Li B, Tesar D, Boswell CA, Cahaya HS, et al. Framework selection can influence pharmacokinetics of a humanized therapeutic antibody through differences in molecule charge. mAbs 2014; 6: 1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.