Abstract
Objectives:
To compare distributions of serum cardiac and inflammatory biomarkers between perinatally HIV-infected (PHIV) and perinatally HIV-exposed uninfected (PHEU) children, to evaluate their associations with echocardiographic measures, and among PHIV youth, with antiretroviral therapy (ART) and HIV disease severity measures.
Design:
Cross-sectional analysis of temporally-paired serum samples for biomarkers and echocardiograms in a prospective cohort study of PHIV and PHEU youth at 14 US pediatric HIV clinics.
Methods:
Serum samples were analyzed among 402 youth in the PHACS Adolescent Master Protocol (AMP) for high-sensitivity cardiac troponin-T (hs-cTnT, a cardiomyocyte injury marker), NT-pro-brain natriuretic peptide (NT-proBNP, a myocardial stress marker), and inflammatory markers (high-sensitivity C-reactive protein, interleukin [IL]-1, IL-6, IL-8, IL-10, IL-18, tumor necrosis factor-α[TNF-α], and soluble TNF receptor II [sTNF-RII]). Echocardiograms were centrally measured and parameters converted to Z-scores to account for differences in age and body size.
Results:
Compared to PHEU (N=156), PHIV youth (N=246) more often had detectable hs-cTnT and higher levels of sTNF-RII and IL-18. Higher inflammatory biomarkers were generally associated with higher left ventricular (LV) wall stress and lower LV function and LV mass in the two groups. Among PHIV youth, the biomarkers were more strongly associated with current rather than historical immunologic and virologic status.
Conclusions:
PHEU and PHIV have modest, significant differences in serum levels of specific inflammatory and active myocardial injury biomarkers. Higher biomarker levels were associated with lower LV mass and shifts in LV structure. Further study is warranted on the longitudinal role of cardiac and inflammatory biomarkers for targeting interventions among PHIV and PHEU youth.
Keywords: HIV, biomarkers, antiretroviral therapy, echocardiography, pediatric
INTRODUCTION
Prior to the widespread use of highly active antiretroviral therapy (HAART), HIV-infected children experienced substantial cardiac morbidity and mortality.1–6 Since the advent of HAART, symptomatic cardiac disease in children has become increasingly rare.7,8 Children in the current era with perinatally-acquired HIV infection (PHIV) have decreased left ventricular (LV) mass but better LV function than PHIV children in the pre-HAART era.9 Lower nadir CD4 percentage and higher HIV viral load were associated with lower LV function (contractility and fractional shortening) and increased LV end-systolic dimension and LV wall stress.9 Subclinical differences in LV structure and function have also been found in perinatally HIV-exposed uninfected (PHEU) children.10–11
In both adults and children, serum biomarkers are useful in assessing adverse changes in cardiac structure and function, termed cardiac remodeling. These biomarkers have been utilized to assess cardiac status in myocardial infarction, cardiomyopathies, heart failure, and myocarditis. Detectable cardiac troponin-T (cTnT) indicates active damage to cardiomyocytes in a variety of cardiac diseases in adults and children.12–18 The level of N-terminal-pro-brain natriuretic peptide (NT-proBNP) varies in response to the LV volume and pressure overloads seen in heart failure and is a marker of myocardial stress and neurohormonal activation.19–23 A recent report in therapy-naïve HIV-infected adults found that moderately elevated NT-proBNP levels were associated with increased HIV viral load and lower CD4 cell counts. These associations were not observed after the initiation of antiretroviral therapy.24 Elevated serum levels of proinflammatory cytokines, (C-reactive protein [hsCRP], tumor necrosis factor-α [TNF-α] and its soluble type 2 receptor [sTNF-RII], interleukin-1 [IL-1], IL-6, IL-8, IL-10, and IL-18), have demonstrated associations with increased cardiovascular mortality and morbidity across a wide age range.25–30
The long-term cardiac effects of antiretroviral therapy (ART) in PHIV children are not well described. A report from the PHACS Surveillance Monitoring for Antiretroviral Therapy Toxicities (SMARTT) PHEU cohort examined the associations between maternal ART regimens and 3 biomarkers (hs-cTnT, NT-proBNP, and hsCRP) and associations between these biomarkers and echocardiographic parameters in a subgroup of the cohort.31 Associations between individual biomarkers and echocardiographic parameters suggested an inflammatory process in the myocardium.
In this study we hypothesized that compared to PHEU youth, PHIV children have higher levels of circulating inflammatory and cardiac biomarkers, and that the levels of these biomarkers would also be associated with alterations in echocardiographic measures of cardiac structure and function. We further hypothesized that in PHIV youth, cardiac biomarkers would be associated with HIV disease severity and ART exposures. Identification of biomarkers of cardiac dysfunction or altered structure among PHIV youths may help in developing effective surveillance systems for future cardiovascular disease during the transition to adulthood, for what has now become a chronic disease.32
METHODS
Study population
The Adolescent Master Protocol (AMP) is a prospective cohort study, conducted by the Pediatric HIV/AIDS Cohort Study (PHACS) at 14 US clinical research sites to evaluate the impact of HIV infection and ART on the outcome of PHIV children and adolescents. The study includes both PHIV children and a control group of PHEU children, aged 7–16 years at study entry, recruited between March 2007 and November 2009. The study protocol was approved by the institutional review board (IRB) of each site and the Harvard T. H. Chan School of Public Health. Written informed consent was obtained from each child’s parent or legal guardian and assent from older participants per local IRB policy.
We compared two study groups: 1) AMP PHIV children who were typically highly treated with ART; and 2) AMP PHEU children who typically were exposed to both prenatal and short-term neonatal ART prophylaxis. Only PHIV and PHEU participants who had a protocol-specified echocardiogram and blood specimen for biomarker analyses within 100 days of each other were included. Children were excluded from analysis if they had a major congenital cardiovascular malformation.
At each AMP study visit, information about participants and their families was gathered through clinical interviews and chart reviews. Current health status was ascertained through physical and laboratory evaluations. The ART regimens were abstracted from medical charts including start and stop dates. HAART was defined as any regimen that included three or more ART drugs from two or more drug classes. The Centers for Disease Control and Prevention (CDC) clinical disease class was determined at study entry and at each study visit according to current pediatric (age <13 years) and adult surveillance definitions.33
Echocardiographic assessment of cardiac structure and function
As per the protocol, PHACS-trained site staff obtained a single M-mode echocardiogram with concurrent blood pressure and heart rate measurements. To improve reliability, all echocardiograms were centrally measured from a digital copy by a single pediatric cardiologist at the echocardiographic core laboratory at Boston Children’s Hospital. Echocardiograms were performed between 2008 and 2010.
Biomarker analyses
All specimens were analyzed for cardiac biomarkers at the Diabetes Research Institute’s Clinical Chemistry Laboratory at the University of Miami Miller School of Medicine. The two cardiac biomarkers, NT-proBNP (an elevated level was defined as >100 pg/mL) and hs-cTnT (limit of detection 3 pg/mL, normal value was defined as undetectable, i.e. <3 pg/mL) were measured by autoanalyzer on a Roche Cobas 6000 by an electrochemiluminescence immunoassay.
Regarding inflammatory biomarkers, hsCRP (elevated level defined as >1mg/L) was measured by latex-enhanced immunoturbidometry using reagents from Roche Diagnostics (Indianapolis, IN). Proinflammatory cytokines (IL-10, IL-1β, IL-6, IL-8, TNF-α, and sTNF-RII) were measured utilizing ELISA kits obtained from R&D Systems (Minneapolis, MN) and IL-18 ELISA from MBL International (Woburn, MA). When available, the high-sensitivity assay kits were used. All manufacturers’ instructions were followed for performing the assays. All samples were analyzed in duplicate, and repeated if the variation between duplicates exceeded 12%.
Statistical Methods
We compared the distribution of baseline characteristics between PHIV and PHEU children using either a T-test or Wilcoxon rank sum test for continuous variables and the Chi-Square test for categorical variables, as appropriate. We also described HIV disease severity and ART measures as current and past CD4 and HIV viral load, current HAART, duration on HAART or protease inhibitor (PI)-based ART, and past AIDS diagnosis based on CDC Class C in PHIV children. We then compared the distribution of serum biomarkers in PHIV to PHEU using Wilcoxon rank sum tests, and compared the percent with elevated serum biomarkers (for hs-cTnT, NT-proBNP, and hsCRP) using Fisher’s exact test.
To evaluate the association of cardiac biomarkers with echocardiographic parameters in PHIV and PHEU children, we calculated partial Spearman correlation coefficients (rs) for each serum biomarker and each echocardiographic Z-score adjusting for age at biomarker assessment, sex, cohort (i.e. PHIV or PHEU), and body-mass index (BMI) Z-score. Partial correlations were also evaluated separately by HIV status and results displayed graphically using heat maps.
Among PHIV youth, we evaluated the association of HIV disease severity and ART exposures with biomarkers by fitting both unadjusted and adjusted linear regression models using generalized estimating equation models with robust standard errors around Z-scores for each log-transformed cardiac biomarker measure, to allow consistent scaling and interpretation of the various biomarkers. All adjusted models controlled for age at biomarker measurement, and additionally controlled for race, ethnicity, sex, and/or BMI Z-score if they were associated with biomarkers (P<0.15). Each HIV disease severity measure was first considered separately, with adjustment for these demographic measures. We then developed multivariable models incorporating multiple HIV disease severity and ART measures associated with each separate biomarker. Due to the high percentage of undetectable hs-cTnT measures, we did not consider linear regression models appropriate and instead fit logistic regression models for elevated hs-cTnT adjusting for demographic measures and again considering each HIV disease severity and ART measure.
SAS version 9.4 was used for all statistical analyses. All statistical tests were two-sided with a P value <0.05 indicating significance, although emphasis was placed on consistency of results across analyses under various assumptions.
RESULTS
Study population
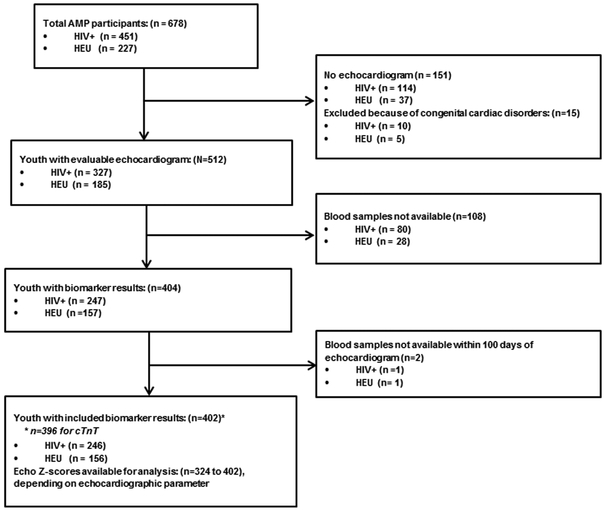
The identification of AMP PHIV and PHEU children for this analysis is described in Figure 1. Overall, of the 512 youth with evaluable echocardiograms, 404 (79%) had evaluable results available for at least one biomarker of interest, and 402 were included in our analysis. The remaining 2 youths did not have a biomarker sample drawn within 100 days of the echocardiogram. The youths included in our analysis represented 55% of the total AMP PHIV cohort and 69% of the PHEU cohort.
Figure 1:
Flow diagram illustrating enrollment and study inclusion.
A summary of participant characteristics for both PHIV and PHEU youth is shown in Table 1. PHIV participants were older on average, more often Black and less often Hispanic, and had lower mean Z-scores for height, weight, BMI and body-surface area (BSA). Among the PHIV youth, over two-thirds had suppressed HIV viral loads (69%), and 87% were actively receiving HAART therapy.
Table 1:
Characteristics of participants in PHACS AMP including HIV disease severity and ART treatment variables, by HIV infection status
| Cohort |
||||
|---|---|---|---|---|
| Characteristic | Perinatally HIV- Infected (PHIV) (N=246) |
Perinatally HIV- exposed Uninfected (PHEU) (N=156) |
Total (N=402) |
P value |
| Age at Biomarker (years), mean (SD) | 13.06 (2.66) | 11.12 (2.58) | 12.30 (2.79) | <0.001 |
| Sex | ||||
| Male | 116 (47%) | 79 (51%) | 195 (49%) | 0.50 |
| Female | 130 (53%) | 77 (49%) | 207 (51%) | |
| Race/Ethnicity | ||||
| Non-Hispanic White | 18 (7%) | 9 (6%) | 27 (7%) | 0.017 |
| Non-Hispanic Black | 158 (64%) | 79 (51%) | 237 (59%) | |
| Hispanic | 60 (24%) | 61 (39%) | 121 (30%) | |
| Other/Unknown | 10 (4%) | 7 (4%) | 17 (4%) | |
| Body measurement Z-scores, mean (SD) | ||||
| Height Z-score | −0.46 (1.26) | 0.20 (1.14) | −0.20 (1.26) | <0.001 |
| Weight Z-score | 0.13 (1.38) | 0.69 (1.34) | 0.35 (1.39) | <0.001 |
| Body-mass index Z-score | 0.37 (1.24) | 0.73 (1.25) | 0.51 (1.25) | 0.006 |
| Body-surface area Z-score | 0.02 (1.31) | 0.50 (1.58) | 0.21 (1.44) | 0.002 |
| Blood pressure Z-scores, mean (SD) | ||||
| Systolic blood pressure | −0.36 (1.05) | −0.38 (1.00) | −0.37 (1.03) | 0.80 |
| Diastolic blood pressure | 0.28 (0.88) | 0.33 (0.78) | 0.30 (0.84) | 0.57 |
| Mean BP Measurement Z-score | 204, −0.54 (0.84) | 136, −0.48 (0.86) | 340, −0.52 (0.85) | 0.53 |
| Echocardiographic Z-scores [N, mean (SD)] | ||||
| LV Function | ||||
| LV Contractility Z-score | 192, 0.18 (0.97) | 132, 0.29 (0.95) | 324, 0.22 (0.96) | 0.29 |
| LV Ejection Fraction Z-score | 241, −0.03 (0.88) | 154, 0.12 (0.89) | 395, 0.03 (0.89) | 0.10 |
| LV Fractional Shortening Z-score | 246, 0.11 (0.93) | 156, 0.32 (0.96) | 402, 0.19 (0.95) | 0.030 |
| LV Structure | ||||
| LV Mass Z-score | 240, 0.34 (0.99) | 153, 0.11 (0.85) | 393, 0.25 (0.95) | 0.014 |
| LV ED Volume Z-score | 241, 0.18 (1.02) | 153, −0.14 (0.98) | 394, 0.06 (1.02) | 0.002 |
| LV ES Volume Z-score | 241, 0.10 (1.01) | 153, −0.18 (0.99) | 394, −0.01 (1.01) | 0.008 |
| LV ED Wall Thickness Z-score | 243, −0.11 (0.83) | 153, −0.22 (0.92) | 396, −0.15 (0.87) | 0.24 |
| LV ED Dimension Z-score | 243, −0.10 (1.02) | 153, −0.48 (0.97) | 396, −0.25 (1.02) | <0.001 |
| LV M-Mode Thickness-to-Dimension Ratio Z-score | 243, −0.32 (0.82) | 154, −0.27 (0.85) | 397, −0.30 (0.83) | 0.55 |
| LV ES Wall Stress Z-score | 201, −1.19 (1.04) | 134, −1.19 (0.98) | 335, −1.19 (1.02) | 0.98 |
| ED Septal Thickness Z-score | 243, −0.38 (0.85) | 153, −0.53 (0.80) | 396, −0.44 (0.83) | 0.092 |
| HIV Disease Severity and Antiretroviral Treatment Measures (PHIV only) | ||||
| CD4 Count <350 cells/mm3 | 25 (10%) | |||
| Median CD4 Count (IQR) | 736 (534,934) | |||
| Nadir CD4% <15% | 84 (34%) | |||
| Suppressed plasma HIV-1 RNA (≤400 copies/mL)* | 169 (70%) | |||
| Peak HIV-1 RNA ≥100,000 copies/mL | 181 (75%) | |||
| ART regimen at assessment | ||||
| HAART with PI | 159 (65%) | |||
| HAART without PI | 54 (22%) | |||
| Non-HAART ARV | 17 (7%) | |||
| Not on ARV | 15 (6%) | |||
| Duration on HAART (years), median (IQR) | 8.93 (6.01, 10.70) | |||
| Duration on PI-based regimen (years), median (IQR) | 8.26 (4.16, 10.62) | |||
| Age at HAART initiation (years), median (IQR) | 2.75 (0.76, 5.53) | |||
| CDC HIV Class C | 60 (24%) | |||
P-values by t-tests (unequal variance) for comparison of Z-scores, Wilcoxon rank sum test for other continuous characteristics, and Chi-square test for categorical characteristics.
Abbreviations: SD=standard deviation, ART=antiretroviral therapy, HAART=highly active antiretroviral therapy, PI=protease inhibitor, CDC=Centers for Disease Control and Prevention, IQR=interquartile range (25th, 75th percentiles).
Some characteristics were not available for all participants: systolic and diastolic blood pressure (N=2 missing), various echocardiographic Z-scores (although all participants had an echocardiographic exam), CD4 count and nadir CD4 percent (N=2), current and peak HIV viral load (N=4), and current ART regimen (N=1).
Comparison of cardiac and inflammatory biomarkers between PHIV and PHEU youth
The distribution of cardiac and inflammatory biomarkers is shown in Table 2 overall and by HIV status. Regarding cardiac biomarkers, the majority of hs-cTnT serum levels were below the limit of detection (3 pg/mL), but a significantly greater proportion of the PHIV youth had an hs-cTnT level >3 pg/mL compared to the PHEU cohort (22% vs. 6%; P<0.001). There was no significant difference between the PHIV and PHEU groups in the median serum level of NT-proBNP or the percent with either elevated NT-proBNP.
Table 2:
Distribution of Serum Biomarkers of AMP Participants by HIV Infection Status
| Cohort |
||||
|---|---|---|---|---|
| Serum Biomarkers | Perinatally HIV- Infected (PHIV) (N=246) Median (IQR) |
Perinatally HIV- exposed Uninfected (PHEU) (N=156) Median (IQR) |
Total (N=402) |
P value* |
| Cardiac Biomarkers | ||||
| NT-proBNP (pg/mL) | 32.55 (13.70, 62.70) | 35.75 (18.00, 60.45) | 34.30 (15.10, 61.00) | 0.33 |
| Abnormal NT-proBNP (>100 pg/mL) | 27 (11%) | 23 (15%) | 50 (12%) | 0.28 |
| Hs-cTnT (pg/mL)** | 3 (3, 3) | 3 (3, 3) | 3 (3, 3) | <0.001 |
| Abnormal hs-cTnT (Above the limit of detection at >3 pg/mL) | 54 (22%) | 10 (6%) | 64 (16%) | <0.001 |
| Inflammatory Biomarkers | ||||
| hsCRP (mg/L) | 0.40 (0.20, 1.30) | 0.50 (0.20, 1.60) | 0.45 (0.20, 1.40) | 0.98 |
| Abnormal hsCRP (>1 mg/L) | 71 (29%) | 48 (31%) | 119 (30%) | 0.74 |
| TNF-α (pg/mL) | 7.70 (4.90, 11.10) | 6.90 (4.70, 9.80) | 7.33 (4.80, 10.60) | 0.20 |
| sTNF-RII (pg/mL) | 2,920 (2,254, 4,057) | 2,612 (2,210, 3,200) | 2,728 (2,236, 3,701) | 0.004 |
| IL-10 (pg/mL) | 2.90 (1.70, 4.80) | 3.00 (1.60, 4.45) | 2.90 (1.60, 4.80) | 0.80 |
| IL-1 (pg/mL) | 0.77 (0.50, 1.50) | 1.00 (0.50, 1.80) | 0.80 (0.50, 1.60) | 0.026 |
| IL-8 (pg/mL) | 6.66 (4.43, 21.90) | 7.10 (4.50, 13.80) | 6.80 (4.47, 19.40) | 0.78 |
| IL-18 (pg/mL) | 260 (196, 356) | 215 (176, 281) | 241 (187, 321) | <0.001 |
| IL-6 (pg/mL) | 1.00 (0.50, 2.10) | 1.16 (0.60, 2.20) | 1.00 (0.60, 2.15) | 0.13 |
P value by Wilcoxon rank sum test.
hs-cTnT values were not measured for 6 participants (4 PHIV, 2 PHEU).
IQR=interquartile range (25th, 75th percentiles); NT-proBNP=N-terminal-pro-brain natriuretic peptide; hsCRP=high-sensitivity C-reactive protein; hs-cTnT=high-sensitivity cardiac troponin-T; TNF-α=tumor necrosis factor-α; sTNF RII=soluble tumor necrosis factor receptor Type II; IL=interleukin.
Regarding inflammatory biomarkers, the median sTNF-RII and IL-18 values were significantly higher in the PHIV cohort than the PHEU cohort. The median IL-1 value was significantly lower in PHIV youth. The distributions of all biomarkers were skewed to the right and were therefore log-transformed in subsequent analyses.
Association of HIV disease severity and ART use with cardiac and inflammatory biomarkers among PHIV youth
Among PHIV children, current immunological and virological status showed stronger associations with cardiac and inflammatory biomarkers than did historical disease severity. After adjusting for potential confounders, we observed an association of low nadir CD4% with higher levels of hsCRP (57% higher for nadir CD4% <15% vs. >15%). Youth with unsuppressed HIV viral loads were more likely to have higher TNF-α, sTNF-RII, IL-10, and IL-18 values, with increases in adjusted mean levels of 31%, 32%, 34%, and 51%, respectively as compared to those with suppressed HIV viral loads (Table 3). Contrary to expectations, youth with unsuppressed HIV viral loads had decreased odds of elevated hs-cTnT, which were attenuated in adjusted models (adjusted odds ratio=0.57, 95% confidence interval: 0.26, 1.25, P=0.16). Finally, youth with peak HIV viral loads ≥100,000 copies/mL had 29% higher average levels of IL-1, while average levels of sTNF-RII were 20% lower in those currently receiving HAART (Table 3).
Table 3:
Association of HIV Disease Severity Measures and Antiretroviral Treatment with Selected Cardiac Biomarkers
| Unadjusted* |
Adjusted* |
||||
|---|---|---|---|---|---|
| HIV Measure | Biomarkers | Change in Biomarker Level (95% CI) |
P value | % Change in Biomarker Level (95% CI) |
P value |
| CD4 T-Cell Count <350 cells/µL | sTNF-RII | 24.5 (7.7, 43.8) | 0.003 | 26.4 (8.5, 47.1) | 0.003 |
| IL-18 | 34.9 (3.4, 76.0) | 0.027 | 37.9 (5.7, 79.9) | 0.018 | |
| Nadir CD4% <15% | NT-proBNP | −20.6 (−38.7, 2.8) | 0.080 | −7.0 (−26.3, 17.4) | 0.54 |
| hsCRP | 60.6 (8.1, 138.7) | 0.019 | 56.8 (3.5, 137.5) | 0.034 | |
| HIV-1 RNA >400 copies/mL | TNF-α | 27.1 (6.2, 52.2) | 0.009 | 30.7 (8.1, 57.9) | 0.006 |
| sTNF-RII | 33.5 (21.0, 47.2) | <0.001 | 31.9 (19.4, 45.7) | <0.001 | |
| IL-10 | 33.8 (10.3, 62.3) | 0.003 | 34.4 (10.7, 63.2) | 0.003 | |
| IL-18 | 48.7 (27.5, 73.6) | <0.001 | 51.0 (29.5, 76.1) | <0.001 | |
| Peak HIV-1 RNA ≥100,000 copies/mL | IL-1 | 30.8 (4.0, 64.5) | 0.021 | 29.2 (3.0, 62.1) | 0.027 |
| Currently on HAART | sTNF-RII | −20.2 (−30.7, −8.1) | 0.002 | −19.9 (−30.5, −7.8) | 0.002 |
| IL-10 | −20.4 (−38.8, 3.7) | 0.091 | −20.3 (−38.9, 3.9) | 0.093 | |
HAART=highly active antiretroviral therapy, CI=confidence interval; NT-proBNP=N-terminal-pro-brain natriuretic peptide; hsCRP=high-sensitivity C-reactive protein; TNF-α=tumor necrosis factor-α; sTNF RII=soluble tumor necrosis factor receptor Type II; IL=interleukin
Table includes only those associations with p<0.10 in either unadjusted or adjusted models. Percent change in biomarker level is based on exponentiating coefficient of GEE linear regression model for log-transformed biomarker for the corresponding HIV disease severity measure or antiretroviral regimen.
All adjusted GEE linear regression models were adjusted for age at biomarker assessment. Additional covariates included in adjusted models were, For NT-proBNP: BMI Z-score, sex, and ethnicity; For hsCRP: BMI Z-score, race, and ethnicity; For TNF-α: sex; For sTNF-RII: BMI Z-score.
Correlation of cardiac and inflammatory biomarkers with echocardiographic measures of LV structure and function
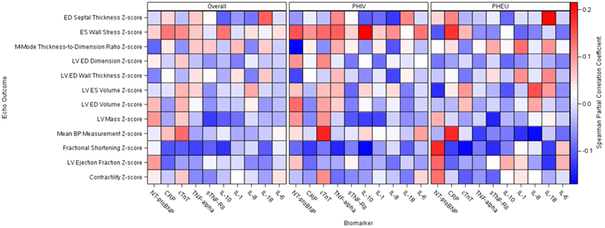
Partial correlation coefficients (e.g. adjusted for covariates) between cardiac and inflammatory biomarkers and echocardiographic Z-scores are displayed graphically using heat maps in Figure 2, both overall and by HIV status. As noted in previous reports, PHIV youth had significantly lower mean Z-scores for LV function (LV fractional shortening), and significantly higher mean Z-scores for LV mass, LV volume, and LV end-diastolic (ED) dimension (Supplemental Table 1). In the combined group of PHIV and PHEU youth, Z-scores for LV function as measured by LV fractional shortening were negatively associated with levels of 6 inflammatory biomarkers (hsCRP, hs-cTnT, TNF-α, sTNF-RII, IL-10, and IL-18), with partial correlations ranging from −0.10 to −0.13. In addition, LV ejection fraction was negatively correlated with hsCRP (r=−0.10), but positively correlated with NT-proBNP levels (r=0.10). Three of the inflammatory biomarkers demonstrated weak negative correlations with LV mass Z-scores (sTNF-RII, IL-10, and IL-1), ranging from −0.09 to −0.13, while NT-proBNP showed a positive correlation with LV mass. LV wall stress was positively correlated with hsCRP, hs-cTnT, and IL-10 (r=0.11 to 0.13).
Figure 2:
A heat map illustrating associations between cardiac and inflammatory biomarkers and echocardiographic parameters, overall and by HIV status. The color scale showing the Spearman partial correlation coefficients is on the right of the figure.
Partial correlation coefficients calculated separately for PHIV and PHEU youth indicate substantial variation by HIV status (Figure 2, Supplemental Tables 2 and 3). Among PHIV participants, the strongest correlation observed was between IL-10 and LV end-systolic wall stress Z-score (rs=0.22), and biomarkers with significant correlations primarily included NT-proBNP, TNF-α, IL-10, and hs-cTnT. Among PHEU children, we observed a weak positive relationship between IL-18 and ED septal Z-score (rs=0.21), and primarily observed associations of echocardiographic measures with NT-proBNP, sTNF-RII and hsCRP. Cardiac and inflammatory biomarkers tended to show much stronger positive associations with LV wall stress among PHIV youth, while they demonstrated stronger negative associations with blood pressure among PHEU youth. Hs-cTnT measures showed stronger positive correlations with all cardiac echocardiographic parameters among PHIV youth, while NT-proBNP was positively correlated with LV function (ejection fraction, fractional shortening, and contractility) only among PHEU youth. In a post-hoc analysis, we investigated potential differences in biomarker-echocardiogram associations by HIV status for ES wall stress and fractional shortening, using linear regression models for echocardiographic z-scores as a function of log-transformed biomarker z-scores and interaction terms with HIV status. We observed that the relation between Ln(NT-proBNP) and fractional shortening z-score was in the opposite direction for PHIV versus PHEU youth (P=0.055). Also, HIV infection status appeared to modify the relationship between Ln(NT-proBNP); (P=0.014), Ln(TNF-alpha); (P=0.068), and Ln(IL-10); (P=0.008) and ES wall stress, with negative associations within PHEU but positive associations among PHIV youth.
DISCUSSION
We observed significant differences in serum levels of several cardiac and inflammatory biomarkers between PHIV and PHEU children. Detectable levels of serum cTnT, a measure of cardiomyocyte injury, were three times as likely to be found in PHIV compared to PHEU children.18 Serum levels of some proinflammatory biomarkers were also higher in PHIV compared to PHEU children, suggesting a modestly higher inflammatory state in the PHIV children even though most were HIV virally suppressed. Compared to an earlier report of younger PHEU from the SMAART cohort, serum levels of NT-proBNP and hsCRP were similar in this older cohort although the percentage with detectable cTnT was lower.31 With the exception of detectable cTnT, all cardiac and inflammatory biomarkers in both the PHIV and PHEU were within normal limits for healthy children although there is variation of reported normal levels in the literature.21,22,34–38 Detectable serum cTnT is never a normal finding in children in the absence of injured cardiac myocytes.17Among PHIV children, the cardiac and inflammatory biomarker levels were more strongly related to current immunological and virological status than with historical disease severity. This finding suggests that the overall goal of keeping viral load as low as possible, preferably at an undetectable level, in PHEU could support cardiac health.
To better understand the potential role of these cardiac and inflammatory biomarkers as markers of cardiac status, we examined their associations with echocardiographic parameters of LV function and structure in PHIV and PHEU children. LV systolic volume was negatively associated with many proinflammatory cytokines. This association supports findings in children with myocarditis, an inflammatory cardiac disorder, where inflammation is associated with proinflammatory cytokines, increased myocardial interstitial edema, and myocardial mononuclear infiltration, resulting in reduced LV volume. Hs-cTnT levels were positively associated with LV end-systolic wall stress, a measure of LV afterload, suggesting active myocardial injury in response to increased LV stress. Alternatively, this association could be operative in the opposite direction, since myocardial injury, as measured by elevated cTnT, can be found with inflammatory myocarditis and is associated with a larger LV dimension and a reduced LV wall thickness, where the presence of either or both may result in increased LV wall stress.
Adverse LV remodeling, indicated by a decreasing LV wall thickness-to-dimension ratio, can evolve overtime. With actute injury, LV dimension rises more rapidly than LV wall thickness due to reduced contractility secondary to acute inflammation. This results in a decreased LV wall thickness-to-dimension ratio, increased LV wall stress, and increased NT-proBNP serum levels (acute dilated physiology). Later, one may see variable LV dilation with attenuated LV and septal wall thickness due to cardiomyocyte injury and fibrosis. These changes are consistent with a chronic low-level inflammatory process and chronically elevated wall stress, although other cardiotoxic mechanisms may contribute.
An analogous, although not identical, example of a cardiotoxic exposure is found in children with cancer exposed to anthracycline-containing chemotherapy regimens.39 Acutely, some of these children will develop a dilated cardiomyopathy and heart failure with increased levels of NT-proBNP and cTnT.40,41 These children have increased LV dimension and LV afterload and decreased LV fractional shortening, mass, and wall thickness. After chemotherapy, up to 65% of children will still have abnormalities of LV structure and function. As they age some children will progress to a restrictive cardiomyopathy with increased LV afterload and decreased LV fractional shortening and mass.39–41 The primary difference in cardiotoxic exposures between PHIV children and childhood cancer survivors is that in the former the potentially cardiotoxic exposure (HIV and/or HAART) is chronic, while in the latter the exposure is relatively transient.
There are many reports of the associations of various serum biomarkers and cardiovascular outcomes in HIV-infected adults. Higher levels of specific proinflammatory biomarkers levels have been reported in HIV-infected adults compared to uninfected adults, are associated with various cardiovascular conditions, and also have been used in cardiovascular disease risk stratification in these adults.34–45 There are few such reports in PHIV or PHEU children.
We previously reported elevated levels of at least one of two cardiac biomarkers (hs-cTnT and NT-proBNP) and one inflammatory biomarker (hsCRP) in PHEU youth, consistent with generalized inflammation and an active myocardial injury process in PHEU children.31 Our current results suggest a proinflammatory state in both PHEU and PHIV children with evidence of more inflammation in the PHIV group. We also found a much higher proportion of PHIV children with evidence of ongoing myocardial damage, as measured by hs-cTnT, compared to PHEU youth. Chronic inflammation is associated with LV diastolic dysfunction and heart failure in HIV-infected children and adults.46–48 In HIV-infected patients with myocarditis, a complex interaction between HIV and other cardiotropic viruses, IL-1, IL-6, TNF-α, and suppression of the anti-inflammatory cytokine IL-10 may explain the cardiotoxic effects leading to dilated cardiomyopathy.49–50
Symptomatic cardiac disease, including LV systolic dysfunction, dilated cardiomyopathy, and heart failure, are now exceedingly rare compared to the pre-HAART era.7 Multiple studies in both PHIV and PHEU children, including this report, have found subclinical cardiac changes whose long-term significance is unknown.9–11,31 Due to these ongoing safety signals we do not know if we are delaying the onset of subsequent symptomatic cardiovascular disease in this PHIV population, instead of having prevented this by current disease management.
Although the rate of perinatal HIV infection has been dramatically reduced, there remain >9500 PHIV children and adults living in the US, as well as 1.8 million PHIV children under the age of 15 years worldwide.51,52 HAART is a global success, but because most treated HIV-infected youth are likely to lead long lives, assessing their risk of cardiovascular disease is important. A recent report emphasized that longitudinal studies evaluating cardiovascular risk in PHIV children are “crucial” and a “research priority”.53 The role of cardiac and inflammatory biomarkers in cardiovascular risk assessment needs to be studied longitudinally to determine their value in cardiovascular risk stratification for PHIV youth.
Strengths and Limitations
The PHACS AMP cohort has large and well-characterized PHIV and PHEU groups with annual evaluations after enrollment The echocardiograms were performed by sonographers trained by the Echocardiography Core Laboratory at Boston’ Children’s Hospital and measured by the pediatric cardiologist who is the director of the laboratory. Samples for serum biomarkers were collected as close to the time of the echocardiogram as possible and measured in a single research clinical chemistry laboratory. The primary limitation of this study is that it is a cross-sectional analysis so causality cannot be inferred from significant associations. Also this design does not allow differentiation between the cardiac effects of chronic HIV infection and exposure to HAART. Finally, the examination of both echocardiograms and serum biomarkers at a single point in time does not provide information about the potential trajectory of cardiac changes over time.
Conclusions
In this study, we found significant, although relatively weak, associations between inflammatory and cardiac biomarkers and decreased LV function and mass, and increased LV wall stress consistent with LV remodeling. These findings are consistent with a chronic inflammatory process, but cannot be confirmed in this cross-sectional study. To determine whether the cardiac changes found in this study are transient, self-limited or represent future cardiac risk in adulthood will require longitudinal cardiac assessments in this population using current cardiac imaging augmented by cardiac magnetic resonance imaging, tissue Doppler, and cardiac strain echocardiography.
Supplementary Material
Acknowledgements:
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart, Lung, and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc. (PI: Julie Davidson).
Source of Funding: The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) with co-funding from the National Institute of Dental & Craniofacial Research (NIDCR), the National Institute Of Allergy & Infectious Diseases (NIAID), the National Institute of Neurological Disorders & Stroke (NINDS), the National Institute on Deafness & Other Communication Disorders (NIDCD), Office of AIDS Research (OAR), the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), and the National Institute on Alcohol Abuse & Alcoholism (NIAAA), through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health or the Department of Health and Human Services.
Source of Funding: National Institutes of Health (HD052102; HD052104)
Reagents for measurement of high-sensitivity cardiac troponin T (hs-cTnT) were provided by Roche Diagnostics Corporation through an Investigator Initiated Research Agreement (No. RD002591).
Footnotes
The following institutions, clinical site investigators and staff participated in conducting PHACS AMP and AMP Up in 2016, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Mirza Baig, Alma Villegas; Children’s Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Patricia A. Garvie, James Blood; Boston Children’s Hospital: Sandra K. Burchett, Nancy Karthas, Betsy Kammerer; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Ray Shaw, Raphaelle Auguste; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson; St. Christopher’s Hospital for Children: Janet S. Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins, Jamie Russell-Bell; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University School of Medicine: Margarita Silio, Medea Gabriel, Patricia Sirois; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Eric Cagwin, Emily Barr, Alisa Katai; University of Miami: Gwendolyn Scott, Grace Alvarez, Gabriel Fernandez, Anai Cuadra.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services.
Conflicts of Interest: All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al. The P2C2 HIV Study Group. Cardiovascular status of infants and children of women infected with HIV-1 (P2C2 HIV): a cohort study. Lancet 2012; 360:368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luginbuhl LM, Orav EJ, McIntosh K, Lipshultz SE. Cardiac morbidity and related mortality in children with symptomatic HIV infection. JAMA 1993; 269:2869–2875. [PubMed] [Google Scholar]
- 3.Lipshultz SE, Easley KA, Orav EJ. Kaplan S, Starc TJ, Bricker JT, et al. for the Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. Cardiac structure and function in children infected with human immunodeficiency virus. The prospective P2C2 HIV multicenter study. Circulation 1998; 97:1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Attar I, Orav EJ, Exil V, Vlach SA, Lipshultz SE. Predictors of cardiac morbidity and related mortality in children with acquired immunodeficiency syndrome. J Am Coll Cardiol 2003; 41:1598–1605. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al. for the Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. Cardiac dysfunction and mortality in HIV-infected children. The prospective P2C2 HIV multicenter study. Circulation 2000; 102:1542–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher SD, Easley KA, Orav EJ, Colan SD, Kaplan S, Starc TJ, et al. Mild dilated cardiomyopathy and increased LV mass predict mortality: The prospective P2C2 HIV Multicenter study. Am Heart J 2005; 150:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher SD, Starc TJ, Guerra V, Williams PL, Wilkinson JD, Lipshultz SE. Declining incidence of systolic LV dysfunction in human immunodeficiency virus-infected individuals treated with highly active antiretroviral therapy. Am J Cardiol. 2016; 117:1194–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel K, Van Dyke RB, Mittleman MA, Colan SD, Oleske JM, Seage GR 3rd; International Maternal Pediatric Adolescent AIDS Clinical Trials 219219C Study Team. The impact of HAART on cardiomyopathy among children and adolescents perinatally infected with HIV-1. AIDS 2012; 26:2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Williams PL, Wilkinson JD, Leister EC, Van Dyke RB, Shearer WT, et al. Cardiac status of HIV-infected children treated with long-term combination antiretroviral therapy: Results from the Adolescent Master Protocol of the NIH Multicenter Pediatric HIV/AIDS cohort study. JAMA Pediatr 2013; 167:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Shearer WT, Thompson B, Rich KC, Cheng I, Orav EJ, et al. Cardiac effects of antiretroviral therapy in HIV-negative infants born to HIV-positive mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children cohort study). J Am Coll Cardiol 2011; 57:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipshultz SE, Williams PL, Zeldow B, Wilkinson JD, Rich KC, van Dyke RB, et al. Pediatric HIV/AIDS Cohort Study (PHACS). Cardiac effects of in-utero exposure to antiretroviral therapy in HIV-uninfected children born to HIV-infected mothers. AIDS 2015; 29:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaze DC, Collinson PO. Multiple molecular forms of circulating cardiac troponin: analytical and clinical significance. Ann Clin Biochem 2008; 45(Pt 4):349–355. [DOI] [PubMed] [Google Scholar]
- 13.Coudrey L The troponins. Arch Intern Med 1998; 158:1173–1180. [DOI] [PubMed] [Google Scholar]
- 14.Ottlinger M, Pearsall L, Rifai N, Lipshultz SE. New developments in the biochemical assessment of myocardial injury in children: Troponin T as highly sensitive and specific markers of myocardial injury. Prog Pediatr Cardiol 1998; 8:71–81. [Google Scholar]
- 15.Lipshultz SE, Rifai N, Sallan SE, Lipsitz SR, Dalton V, Sacks DB, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 1997; 96:2641–2648. [DOI] [PubMed] [Google Scholar]
- 16.Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med 2004; 351:145–153. [DOI] [PubMed] [Google Scholar]
- 17.Lipshultz SE, Wong JC, Lipsitz SR, Simbre VC 2nd, Zareba KM, Galpechian V, et al. Frequency of clinically unsuspected myocardial injury at a children’s hospital. Am Heart J 2006; 151:916–922. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch R, Landt Y, Porter S, Canter CE, Jaffe AS, Ladenson JH, et al. Cardiac troponin I in pediatrics: normal values and potential use in the assessment of cardiac injury. J Pediatr 1997; 130:872–877. [DOI] [PubMed] [Google Scholar]
- 19.Ruskoaho H Cardiac hormones as diagnostic tools in heart failure. Endocr Rev 2003; 24:341–356. [DOI] [PubMed] [Google Scholar]
- 20.Mangat J, Carter C, Riley G, Foo Y, Burch M. The clinical utility of brain natriuretic peptide in paediatric LV failure. Eur J Heart Fail 2009; 11:48–52. [DOI] [PubMed] [Google Scholar]
- 21.Ratnasamy C, Kinnamon DD, Lipshultz SE, Rusconi P. Associations between neurohormonal and inflammatory activation and heart failure in children. Am Heart J 2008; 155:527–533. [DOI] [PubMed] [Google Scholar]
- 22.Rusconi PG, Ludwig DA, Ratnasamy C, Mas R, Harmon WG, Colan SD, et al. Serial measurements of serum NT-proBNP as markers of LV systolic function and remodeling in children with heart failure. Am Heart J 2010; 160:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol 2012; 30:1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuster C, Binder C, Strassl R, Aichelburg MC, Blackwell E, Pavo N, et al. Impacet of HIV infection and antiretroviral treatment on N-terminal prohormone of brain natriuretic peptide as surrogate of myocardial function. AIDS 2017; 31:395–400. [DOI] [PubMed] [Google Scholar]
- 25.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Framingham Heart Study. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation 2003; 107:1486–1491. [DOI] [PubMed] [Google Scholar]
- 26.Yan AT, Yan RT, Cushman M, Redheuil A, Tracy RP, Arnett DK, et al. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the Multi-Ethnic Study of Atherosclerosis. Eur Heart J 2010; 31:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh P, Murray HM, Ford I, Trompet S, de Craen AJM, Jukema JW, et al. for the PROSPER Study Group. Circulating interleukin-10 and risk of cardiovascular events: A prospective study in the elderly at risk. Arterioscler Thromb Vasc Biol 2011; 31:2338–2344. [DOI] [PubMed] [Google Scholar]
- 28.Pye M, Rae AP, Cobbe SM. Study of serum C-reactive protein concentration in cardiac failure. Br Heart J 1990; 63:228–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller TL, Orav EJ, Lipshultz SE, Arheart KL, Duggan C, Weinberg GA, et al. Risk factors for cardiovascular disease in children infected with human immunodeficiency virus-1. J Pediatr 2008; 153:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 2002; 106:24–30. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson JD, Williams PL, Leister E, Zeldow B, Shearer WT, Colan SD, et al. Cardiac biomarkers in HIV-exposed uninfected children: the Pediatric HIV/AIDS Cohort Study (PHACS). AIDS 2012; 29:91–100. [Google Scholar]
- 32.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents and young adults with perinatally acquired HIV infection. Annu Rev Med 2010; 61:169–185. [DOI] [PubMed] [Google Scholar]
- 33.AIDS Education and Training Centers National Resource Center. HIV Classification: CDC and WHO Staging Systems. April 2014. https://aidsetc.org/guide/hiv-classification-cdc-and-who-staging-systems. Accessed December 17, 2016.
- 34.Monsuez JJ, Escaut L, Teicher E, Charniot JC, Vittecoq D. Cytokines in HIV-associated cardiomyopathy. Int J Cardiol 2007; 120:150–157. [DOI] [PubMed] [Google Scholar]
- 35.Baker JV, Duprez D. Biomarkers and HIV-associated cardiovascular disease. Cur Opin HIV AIDS 2010; 5:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jalbert E, Crawford TQ, D’Antoni ML, Keating SM, Norris PJ, Nakamoto BK, et al. IL-1B enriched monocytes mount massive IL-6 responses to common inflammatory trigger among chronically HIV-1 infected adults on stable antiretroviral therapy at risk for cardiovascular disease. PLoS ONE. 2013; 8:e75500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McComsey GA, Kitch D, Sax PE, Tierny C, Jahed NC, Melbourne K, et al. Associations of inflammatory markers with AIDS and non-AIDS clinical events after initiation of antiretroviral therapy: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Acquir Immune Defic Syndr 2014; 65:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Secemsky EA, Scherzer R, Nitta E, Wu AHB, Lange DC, Deeks SG, et al. Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in HIV-infected individuals. JACC Heart Fail 2015; 3:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Hear. 2008; 94:525–533. [DOI] [PubMed] [Google Scholar]
- 40.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 1995; 332:1738–1743. [DOI] [PubMed] [Google Scholar]
- 41.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2005; 23:2629–2636. [DOI] [PubMed] [Google Scholar]
- 42.Ntusi N, O’Dwyer E, Dorrell L, Wainwright E, Piechnik S, Clutton G, et al. HIV-1-related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging 2016; 9:e004430. [DOI] [PubMed] [Google Scholar]
- 43.So-Armah KA, Tate JP, Chang CH, Butt AA, Gerschenson M, Gilbert CL, et al. Do biomarkers of inflammation, monocyte activation, and altered coagulation explain excess mortality between HIV infected and uninfected people? J Acquir Immune Defic Syndr 2016; 72:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection. A systematic review. PLos One 2016; 11:e014784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guzman-Fulgencio M, Medrano J, Rallon N, Echeverria-Urabayen A, Miguel Benito J, Restropo C, et al. Soluble markers of inflammation are associated with Framingham scores in HIV-infected patients on suppressive antiretroviral therapy. J Infect 2011; 63:382–390. [DOI] [PubMed] [Google Scholar]
- 46.Guerra VC, Leister EC, Williams PL, Starc TJ, Lipshultz SE, Wilkinson JD, et al. Long-term effects of in utero antiretroviral exposure: systolic and diastolic function in HIV-exposed uninfected youth. AIDS Res Hum Retroviruses 2016; 32:621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manga P, McCutcheon K, Tsabedze N, Vachiat A, Zachariah D. HIV and nonischemic heart disease. J Am Coll Cardiol. 2017; 69:83–91. [DOI] [PubMed] [Google Scholar]
- 48.Barnes RP, Lacson JC, Bahrami H. HIV infection and risk of cardiovascular diseases beyond coronary artery disease. Curr Atheroscler Rep 2017; 19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher SD, Bowles NE, Towbin JA, Lipshultz SE. Mediators in HIV-associated cardiovascular disease: a focus on cytokines and genes. AIDS 2003; 17 Suppl 1:S29–35. [DOI] [PubMed] [Google Scholar]
- 50.Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis 2006; 185:1–11. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. HIV Among Pregnant Women, Infants and Children. Atlanta, GA: Centers for Disease Control and Prevention; 2016. Accessed from: http://www.cdc.gov/hiv/group/gender/pregnantwomen/. [Google Scholar]
- 52.UNAIDS. AIDSInfo. Accessed from http://aidsinfo.unaids.org/ on May 31, 2017.
- 53.Dirajlal-Fargo S, Sattar A, Kulkarni M, Bowman E, Funderburg N, McComsey GA. HIV-positive youth who are perinatally infected have impaired endothelial function. AIDS 2017; 31:1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.