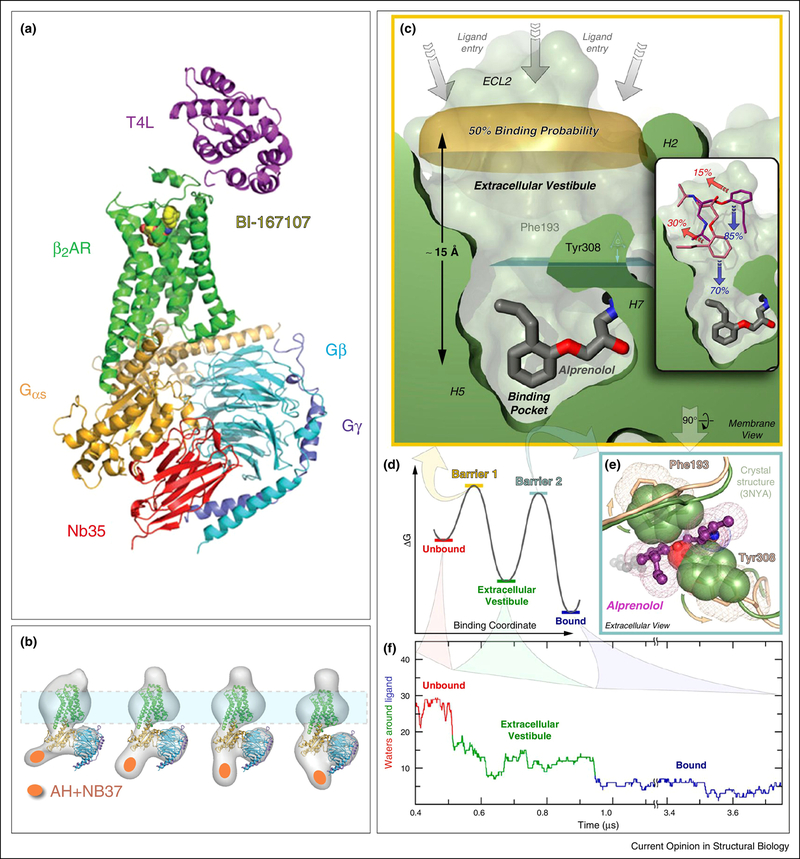
Figure 3. Structure and dynamics of a GPCR-G protein complex.
A) X-ray structure [80] of agonist (yellow spheres) bound β2AR (green) interacting with the heterotrimeric G protein Gs, composed of Gαs (orange), which has an extensive binding surface with the β2AR, and Gβ (cyan) and Gγ (purple). The Nb35 nanobody (red) binds between Gα and Gβ, and T4 lysozyme (magenta) was expressed as a fusion protein at the β2AR N-terminus. Nb35 and T4 lysozyme facilitated crystallization. B) Fitting the X-ray crystal structure of T4L-β2AR in A) into 2D projections of particle class averages imaged by EM [83]. The Nb37 nanobody (orange oval) binds to the α-helical (AH) domain of Gsα and was used in the EM experiments to visualize the conformational flexibility of the AH domain. (C-F) MD simulation of antagonist binding [89]. C) Extracellular vestibule and orthosteric binding pocket of β2AR in relation to the antagonist alprenolol. D) Energy barriers of binding alprenolol to the extracellular vestibule and the orthosteric binding site E) Residues impeding access to the orthosteric binding site F) Microsecond simulation of dehydration of alprenolol during binding to the β2AR in a ~50,000 atom system.