FIGURE 1.
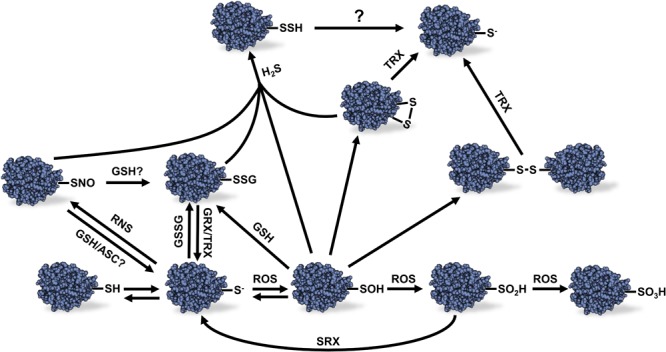
Different redox modifications of protein Cys thiols and their reversibility. Protein thiol oxidation can lead to a variety of redox modifications. The scheme presents the relations between the different redox modifications and the reversibility of these modifications. Sulfonic acid (–SO3H) formation is considered to be irreversible as well as sulfinic acid (–SO2H) except in the case of certain peroxiredoxins which sulfinic acid Cys residue can be reduced by sulfiredoxin using and ATP-dependent mechanism. See text for details. ROS, reactive oxygen species; RNS, reactive nitrogen species; H2S, hydrogen sulfide; GSSG, oxidized glutathione; GSH, reduced glutathione; ASC, ascorbate; GRX, glutaredoxin; TRX, thioredoxin; SRX, sulfiredoxin; R-SH, reduced thiol; R-S-, thiolate anion; R-SOH, sulfenic acid; R-SNO, protein S-nitrosylation; R-SSG, protein S-glutathionylation Cys; R-SSH, protein S-sulfhydration; R-SS-R, disulfide bond.
