In response to rising rates of opioid overdose and addiction, growing numbers of patients with opioid use disorder are being transitioned from Schedule II prescription and illicit opioids to buprenorphine or buprenorphine/naloxone. From 2010 to 2016, annual prescriptions for buprenorphine products more than doubled, while other categories of opioid prescriptions remained level or decreased. Physicians are faced with the clinical dilemma of what to do when patients on buprenorphine need surgery.
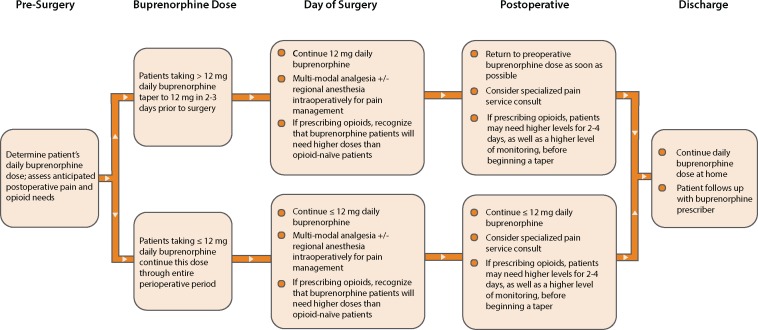
In the absence of convincing evidence that continuing buprenorphine leads to poorer outcomes, and in light of the risks associated with discontinuing buprenorphine, we recommend that patients with opioid use disorder on buprenorphine therapy continue the medication throughout the peri-operative period, as well as in situations requiring urgent/emergent analgesia. For patients on higher doses of buprenorphine (more than 12 mg daily) scheduled to undergo painful procedures such as total joint replacement or open abdominal surgery, we have developed a protocol to lower the dose but still maintain buprenorphine through the peri-operative period (Figure 1).
Figure 1.
Perioperative Buprenorphine Protocol.
Buprenorphine is a Schedule III opioid that is Food and Drug Administration (FDA) approved for the treatment of opioid use disorder and pain (in some formulations). Because of its unique pharmacologic properties, it poses lower risks of misuse, respiratory suppression, and accidental overdose. Buprenorphine is also a partial agonist: due to tight binding at the mu opioid receptor and attenuated intrinsic activity, buprenorphine blocks some effects of other opioids a patient may take or be prescribed. Therefore, whether to continue buprenorphine when using other opioids to manage acute or peri-operative pain has become an issue of controversy and public health significance.
Published guidelines and opinions in the United States recommend discontinuing buprenorphine well in advance of anticipated pain or major surgery [1,2]. The US Center for Substance Abuse Treatment (CSAT) in its 2004 Treatment Improvement Protocol (TIP) stated, “While patients are taking opioid pain medications, the administration of buprenorphine generally should be discontinued” [2]. The 2004 CSAT guidelines have had an outsized influence on medical practice, and the discontinuation of buprenorphine prior to surgery has become the de facto standard of care. These recommendations derive from published cases of difficult-to-treat acute pain in buprenorphine-maintained patients; but these case reports are not universally persuasive. They may primarily reflect the challenge of managing already opioid-tolerant and -dependent patients in need of analgesia, as opposed to difficulties related to buprenorphine per se.
Furthermore, clinical pain research dating even as far back as 1990 shows buprenorphine in combination with other opioids effectively treating peri-operative or other acute pain [3–6]. In 2013, a group of Australian clinicians at the Royal Adelaide Hospital published a comparison between 22 buprenorphine and 29 methadone-maintained patients undergoing elective major surgery and found that the buprenorphine patients required less intravenous breakthrough pain medication in the first 24 hours postoperatively. They concluded, “These results confirm that continuation of buprenorphine peri-operatively is appropriate” [4]. A study in 2017 compared post–cesarean section opioid analgesic pain requirements in women with opioid use disorder treated with methadone or buprenorphine. The study found no differences between groups in length of hospital stay, postoperative complications, or need for opioid analgesia [6].
Beyond the data, we believe discontinuing buprenorphine prior to surgery in patients with opioid use disorder introduces unnecessary risk for four reasons:
Discontinuing buprenorphine introduces management complexity by delaying surgery to allow adequate time to taper, requiring more clinic visits and care coordination between multiple providers (primary prescriber, surgeon, and anesthesiologist), and burdening patients with additional preoperative instructions and tasks.
Re-induction onto buprenorphine after surgery is likely to be physically painful and medically destabilizing for patients because it forces them to endure a period of active opioid withdrawal before buprenorphine can be restarted.
Re-induction onto buprenorphine, particularly in the outpatient setting, is logistically complicated and labor intensive, and the potential for discontinuity in care and patient nonadherence looms large, especially when multiple prescribers are involved. It also places an undue burden on outpatient buprenorphine prescribers, already straining to meet increasing demand.
Patients on buprenorphine for opioid use disorder are at increased risk for relapse to opioid misuse and accidental overdose when buprenorphine is discontinued because they experience an opioid deficit while simultaneously gaining access to a new supply of full-opioid agonists.
Whether to continue the patient’s current dose of buprenorphine or taper them to a lower dose but not off, to provide more mu receptor availability, has not been studied. Nonetheless, we recommend that patients taking 12 mg or less of buprenorphine daily maintain this dose unchanged, and patients on higher daily doses of buprenorphine (more than 12 mg) taper to 12 mg two to three days prior to surgery (Figure 1).
The recommendation to taper patients, when possible, down to 12 mg buprenorphine is based on studies of receptor occupancy and clinical experience. Receptor binding studies utilizing positron emission tomography scanning and radiolabeled (11C) carfentanil in buprenorphine-treated heroin-addicted persons confirm a dose response curve of reduced but conserved availability of mu opioid receptors in patients maintained on varied doses of daily buprenorphine: 100% at 0 mg, 59% at 2 mg, 20% at 16 mg, and 16% at 32 mg [7].
Clinical experience suggests that, as with any patient taking varied doses of opioids prior to surgery, patients maintained on buprenorphine may proportionally need more opioids for postoperative pain, as compared with opioid-naïve patients. Some may require cardiac and oximetry monitoring along with multimodal analgesia, including intravenous ketamine. But we have found that tapering patients, when possible, down to 12 mg buprenorphine for two to three days prior to surgery is clinically uncomplicated and addresses the potential challenge of the reduction of available mu opioid receptors.
Tapering patients too far in advance of surgery may cause both discomfort and increased risk for relapse, which is why we recommend the taper be done no more than a few days before surgery. In cases where clinical judgment suggests that tapering to 12 mg would constitute a significant risk of relapse, or where clinical presentation necessitates emergent surgery (e.g., the acute trauma patient), it is acceptable to continue the dose up to the FDA-recommended limit of 24 mg daily.
We recommend continuing the dose of buprenorphine at 12 mg or less for one to three days after surgery should other opioids be needed for breakthrough pain, after which buprenorphine can be returned to its preoperative dose and the other opioids tapered in as timely a way as possible. Involving an acute pain service in the management of these patients is ideal. Analgesia is challenging in this patient population, and co-management with pain specialty services may help with pain control, buprenorphine dosing, and timely patient discharge. Communication prior to discharge between inpatient and outpatient treatment providers is key to the successful management of these patients. Postoperative planning is optimized by preoperative care coordination.
We recognize that discontinuing buprenorphine completely may be a better option for some patients and support the personalization of pain medicine for each individual. But we nonetheless believe that the practice of routinely discontinuing buprenorphine in this setting is misguided. Maintaining buprenorphine through the peri-operative period in patients being treated for opioid use disorder avoids risk of relapse and undue burden on the health care system and promotes safety, adherence, and continuity of care.
Disclosure and conflicts of interest: No conflicts of interest. Dr. Ottestad serves on the scientific board and as a consultant for Bioness (nerve stimulators).
Acknowledgments
We acknowledge the members of the Stanford Pain and Addiction Collaborative who participated in a meeting addressing the problem of buprenorphine discontinuation in the peri-operative period.
References
- 1. Anderson TA, Quaye ANA, Ward EN, et al. To stop or not, that is the question: Acute pain management for the patient on chronic buprenorphine. Anesthesiology 2017;1266:1180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Center for Substance Abuse Treatment Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Metnal Health; 2004. [Google Scholar]
- 3. Kornfeld H, Manfredi L.. Effectiveness of full agonist opioids in patients stabilized on buprenorphine undergoing major surgery: A case series. Am J Ther 2010;175:523–8. [DOI] [PubMed] [Google Scholar]
- 4. Macintyre PE, Russell RA, Usher KAN, Gaughwin M, Huxtable CA.. Pain relief and opioid requirements in the first 24 hours after surgery in patients taking buprenorphine and methadone opioid substitution therapy. Anaesth Intensive Care 2013;412:222–30. [DOI] [PubMed] [Google Scholar]
- 5. Silva MJ, Rubinstein A.. Continuous perioperative sublingual buprenorphine. J Pain Palliat Care Pharmacother 2016;304:289–93. [DOI] [PubMed] [Google Scholar]
- 6. Vilkins AL, Bagley SM, Hahn KA, et al. Comparison of post-cesarean section opioid analgesic requirements in women with opioid use disorder treated with methadone or buprenorphine. J Addict Med 2017;115:397–401. [DOI] [PubMed] [Google Scholar]
- 7. Greenwald MK, Johanson C, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology 2003;2811:2000–9. [DOI] [PubMed] [Google Scholar]