Abstract
Background
Renal transplantation is the treatment of choice for chronic kidney disease (CKD) patients, but the shortage of kidneys and the disabling medical conditions these patients suffer from make dialysis essential for most of them. Since dialysis drastically affects the patients' lifestyle, there are great expectations for the development of wearable artificial kidneys, although their use is currently impeded by major concerns about safety. On the other hand, dialysis patients with hemodynamic instability do not usually tolerate intermittent dialysis therapy because of their inability to adapt to a changing scenario of unforeseen events. Thus, the development of novel wearable dialysis devices and the improvement of clinical tolerance will need contributions from new branches of engineering such as artificial intelligence (AI) and machine learning (ML) for the real-time analysis of equipment alarms, dialysis parameters, and patient-related data with a real-time feedback response. These technologies are endowed with abilities normally associated with human intelligence such as learning, problem solving, human speech understanding, or planning and decision-making. Examples of common applications of AI are visual perception (computer vision), speech recognition, and language translation. In this review, we discuss recent progresses in the area of dialysis and challenges for the use of AI in the development of artificial kidneys.
Summary and Key Messages
Emerging technologies derived from AI, ML, electronics, and robotics will offer great opportunities for dialysis therapy, but much innovation is needed before we achieve a smart dialysis machine able to analyze and understand changes in patient homeostasis and to respond appropriately in real time. Great efforts are being made in the fields of tissue engineering and regenerative medicine to provide alternative cell-based approaches for the treatment of renal failure, including bioartificial renal systems and the implantation of bioengineered kidney constructs.
Keywords: Hemodialysis, Artificial kidney, Artificial intelligence, Machine learning
Introduction
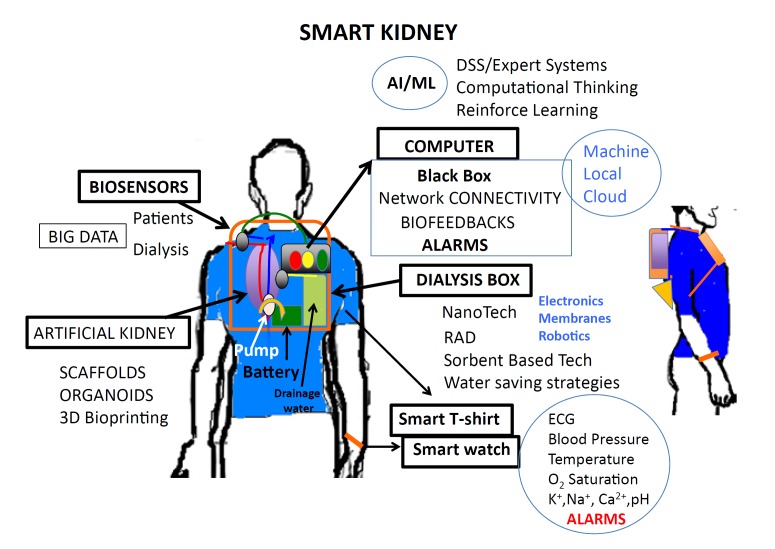
Hemodialysis is a renal replacement therapy based on the use of an artificial kidney (dialyzer) that removes waste products, chemical substances, and fluids from blood. In the field of dialysis, there has been large progress in hemodynamic tolerability by means of biofeedback-assisted devices, and there is currently a focus on portable, wearable devices and self-care systems to improve the quality of life of renal patients (Fig. 1).
Fig. 1.
Artificial intelligence involves the science and engineering for developing smart wearable artificial kidneys.
Emerging technologies derived from artificial intelligence (AI) and machine learning (ML) are increasingly transforming medical procedures and devices and will offer great opportunities for dialysis therapy. The most recent trends in the development of computer-based intelligent decision support systems (DSS) and expert systems applied to dialysis are based on artificial neural networks (ANN) and genetic algorithms that must learn their knowledge interactively from the users. The ongoing explosion of interest in deep learning, an expanded and complex version of shallow ANN, is also being experienced in the medical field. These methods have, for instance, been used in image diagnosis [1] and in alerting systems and diagnostic assistance [2, 3, 4]. The efficacy of machine intelligence improves with the quantity of data it consumes, since it is based on algorithms that learn from previous data-based experiences. Thus, the internet of things (IoT), defined as a system of interrelated computing devices with the ability to transfer data over a network without necessarily requiring human-to-human or human-to-computer interaction, has emerged as a powerful data-generating environment that AI can use to obtain data insights and extract novel and usable knowledge [5]. This environment is of great relevance to clinical practice. The more data involved in these IoT systems (for example Watson IoT Platform or the IoT Accelerator platform from Ericsson), the more AI algorithms can perfect their ability to detect global and local patterns and anomalies, identify medical conditions, or even issue alerts based on such information [6].
However, much innovation on AI and ML is needed before we could achieve a smart dialysis machine able to analyze and understand changes in patient homeostasis and respond appropriately in real time. The technological advancements required include machine size, network connectivity (predominantly in the form of the IoT), and computing efficiency. In addition, these resources need to be robust, fault tolerant, and cost efficient. For this purpose, device size is still a relevant factor, because only the large ones are currently able to store the amounts of data required for continuous learning. On the other hand, miniaturized portable devices must constantly download data for DSS and expert system. Thus, connectivity is essential for accessing large amounts of data, and small dialysis devices will require continuous and robust connectivity in order to ensure continuous patient monitoring and treatment optimization. Nevertheless, current developments in computer chip miniaturization and speed will surely facilitate the development of autonomous dialysis devices without requiring the download of data.
The aim of this review is to provide an update on the current progress in artificial kidneys, biosecurity devices, and AI, as well as to point out the challenges for the future development of wearable devices presented at the “2nd Meeting of Science and Dialysis: Artificial Intelligence,” organized at the Hospital Universitari Bellvitge, Barcelona, Spain. Since there are 2 main scenarios in nephrology current clinical practice, namely acute patients with hemodynamic instability and multiple organ failure who need renal replacement therapy and chronic patients with end-stage renal disease on dialysis, we will analyze current progress and challenges separately.
Acute Hemodynamically Unstable Patients Require a Continuous Assessment of the Treatment Prescription
Critically ill patients with hemodynamic instability and acute kidney injury (AKI) usually do not tolerate intermittent dialysis therapy. Their most frequent complication is hypotension caused by hypovolemia, not compensated by the intravascular refilling rate from the interstitial compartment, with the consequence of an increase in hemodynamic instability. The arterial response to hypovolemia is a complex interplay of active and passive mechanisms, including a decreased venous vessel capacity to sustain cardiac filling; increased arterial vascular resistances to ensure organ perfusion, and increased myocardial contractility and heart rate to maintain cardiac output [7]. Thus, continuous renal replacement therapies (CRRT) are the choice in hemodynamically instable patients, because they allow a slow and constant fluid removal, targeted to match the plasma refill rate, to ensure patient survival and renal functional recovery. These techniques of renal substitution therapy have allowed the conceptual shift from renal “replacement” to renal “support” therapies [8], and the term “dynamic CRRT” has been proposed to show that any treatment has to be dynamic and adaptable to the constantly changing clinical status of critically ill patients [9].
The delivery phase of CRRT is today supported by technological improvements in hardware and software that provide guidance on several aspects of the treatment, including downtime dose compensation, calcium replacement during citrate anticoagulation, circuit pressure profiles, and appropriate fluid balance. Furthermore, arranging large screens on machines would permit the downloading of online tutorials and assisted troubleshooting [10]. These new technological advances provide the basis for an individualized therapy according to specific clinical conditions and patient needs.
Challenges of Supportive Therapies in Critically Ill Patients and Predictions about Future Dialysis Devices
The therapeutic goals in critically ill patients with AKI are the maintenance of fluid and electrolyte balance, an adequate nutrition, and the treatment of infection when present. However, mortality in critically ill patients is still high [11], and attempts to improve AKI outcomes by increasing the dose of hemodialysis or hemodiafiltration have been disappointing [12]. Therefore, considering that only increasing the clearance of small solute shows a minimal impact on clinical outcomes, it has been suggested that renal tubular functions (reabsorption and secretion of solutes, metabolic functions, and hormone production) could have key roles in replacement therapy to improve survival [13, 14]. Thus, a renal tubule assist device (RAD) containing living renal proximal tubules was successfully engineered and demonstrated differentiated absorptive, metabolic, and endocrine functions of normal kidney both in in vitro and ex vivo animal models [15]. So far, RAD remains the only viable bioartificial kidney device that has been used in patients with AKI receiving CRRT [14]. However, a follow-up phase IIb trial had to be stopped because of an unexpectedly high survival rate in patients treated with control sham RAD without seeded cells [16]. Additional therapeutic approaches include the Bioartificial Renal Epithelial Cell System (BRECS) which had promising results in extracorporeal large animal studies [17] but has not yet been tested in patients.
Currently, the technical limitations of the equipment for supporting CRRT include the software for hemodynamic optimization, the need for a continuous evaluation of the efficiency of solute and fluid removal, and the management of big amounts of data [8]. The continuity of the management of AKI should include biofeedback for dialysis prescription, but we have to bear in mind that the speed of this feedback will depend on the frequency of data acquisition and evaluation. Thus, a desired technical improvement of dynamic CRRT would be the availability of online tools for continuous, real-time data acquisition from the machine and from patients' electronic medical records to establish an automatic biofeedback loop [10]. Monitoring and data collection systems based on various connectivity platforms (machine, local, or cloud-based) will help nephrologists to evaluate whether end points of the delivered treatment are achieved and support modifications of the initial CRRT prescription based on updated clinical targets. Recent advancements in AI technology and sensing have been used in intensive care units to predict which patients are at the highest risk of imminent deterioration and to alert staff [18] and for monitoring critically ill patients and their environment using wearable sensors, namely light and sound sensors and a high-resolution camera [19]. However, AI technologies face ethical and legal challenges yet to clarify. Google DeepMind launched a pivotal project in 2016 to develop a clinical alert smartphone app that functions as a data-integrating user interface to check test results for signs of AKI. The aim was to build a “real time clinical analytics, detection, and diagnosis and decision support to support treatment and avert clinical deterioration across a range of diagnoses and organ systems” [20]. However, the project was halted due to a lack of privacy and consent to transferring population-derived datasets to large private prospectors [21].
The challenges for future dialysis devices include the design of smart dialysis software that automatically takes “smart” decisions. As previously mentioned, DSS and expert systems need previous experience to infer about and solve problems. But, how are experts to quickly solve a problem in which they have no previous experience in an efficient and timely manner? An answer to these challenges could come from the Computational Thinking field that is defined as the thought processes involved in formulating a problem and expressing its solutions in such a way that a computer can effectively carry out [22]. Computational Thinking is an iterative process based on 3 stages: (1) abstraction (problem formulation); (2) automation (solution expression), and (3) analyses (solution execution and evaluation). Thus, the characteristics are decomposition, pattern recognition/data representation, generalization/abstraction, and algorithms. By decomposing a problem, identifying the variables involved using data representation and creating algorithms, a generic solution will result. The generic solution is a generalization or abstraction that can be used to solve a multitude of variations of the initial problem. The idea of abstraction (to hide the details and focus on the important) requires recognizing patterns that allow us to transform complex problems into simple models. Concealing layers of information makes it possible to get at the intersections of things, improving aspects of a complicated system without the need to understand each part. Abstractions allow advances without starting from the beginning [23]. The potential problems of this approach include how to handle and control potential errors, uncertainty, and systematic bias.
To conclude this section, it is necessary to say that CRRT is delayed regarding the technology of miniaturization, although the incorporation of nanotechnology holds promise for enhancing the portability of future devices. In addition, the use of sorbent-based techniques and water-saving strategies are also potential approaches for the development of miniaturized technologies by reducing the volume of effluent.
The Challenge for Chronic Hemodialysis Patients Is the Engineering of Artificial Kidneys
Great efforts are being made in the fields of tissue engineering and regenerative medicine to provide alternative cell-based approaches for the treatment of renal failure, including bioartificial renal systems and the implantation of bioengineered kidney constructs [24]. The basic strategy of bioengineered kidney constructs is to exploit the structure of the native kidney through seeding cultured cells into a 3D scaffold system and subsequently implant the cell-seeded scaffold in vivo to restore kidney functions. Various scaffolding systems, including natural (e.g., collagen) and synthetic biomaterials, have been used, and recent advances have been made in the generation of kidney organoids in vitro that engraft in vivo [25, 26]. Although promising, these technologies are still far from clinical application due to difficulties in the fabrication of large-sized functional renal constructs with complex renal structures that could readily integrate into host kidney tissue for clinical translation [27]. Furthermore, complex organs require an intact vascular network that can be further reconnected to the circulatory system to ensure adequate nutrients and oxygen.
One of the main problems of transplantation of recellularized whole kidney scaffolds is the need to efficiently repopulate the endothelium of the vascular network of the engineered kidney before implantation in order to avoid thrombosis [28]. Another unresolved issue is the optimal source of cells to repopulate an acellular kidney scaffold. However, it has been suggested that pluripotent stem cells-derived progenitor renal cells rather than more mature renal cell phenotypes would be the most appropriate to produce a bioengineered kidney [27]. Thus, the scaling up of the technology to recellularize human size kidney scaffolds will require improved cell differentiation protocols, efficient cell delivery strategies, and innovative organ bioreactor systems with physiologically relevant kidney culture conditions.
An alternative in tissue engineering to whole kidney scaffold is the use of 3D bioprinting of living organ-like structures. 3D bioprinting is a promising additive manufacturing technology based on the deposition of biomaterials and cells in micrometer scale (with a printing resolution of 10–10,000 μm) to form precise structures comparable to tissue [29]. In most cases, a 3-axis mechanical platform controls the movement of extruders printing the bioink in the required shape according to an algorithm. In the context of kidney regeneration, the successful generation of functional kidney scaffolds by 3D bioprinting is limited by the possibility of mimicking the composition and spatial organization of kidney extracellular matrix and the ability to implant accurately different renal cell populations in an organized manner. Thus, several challenges need to be taken into account in the design and implementation of 3D bioprinting techniques to construct a whole engineered kidney useful for patients, such as the development of novel biocompatible bioinks and biomaterials with fast crosslinking properties; the improvement of bioprinting manufacturing devices with enough spatial resolution to recapitulate the hierarchical structure in kidneys and the fidelity of printing; the stability and mechanical properties of the bioprinted kidney to ensure successful transplantation; the need for vascularization; and the development of adapted perfusion bioreactors suitable for organ printing and further maturation [29].
One remarkable innovation is the implantable Renal Assist Device (iRAD) that uses micromachining techniques to fabricate a biohybrid system able to mimic renal morphology and function. This artificial kidney is a bionic device that incorporates a silicon nanopore membrane and a bioreactor of live kidney cells that will concentrate the ultrafiltrate into urine. The bundle is enclosed in a body-friendly box and connected to a patient's circulatory system and bladder. Despite having been used with success in animal models [30], it is still under development to scale it up to the clinical environment [31]. Challenges for the implantable artificial kidney include cell sourcing, organ scaffolding, immune response, package size of the dialyzer, clotting [32], and water requirements for preparation of the dialysate.
Another interesting approach is the wearable artificial kidney, a 5-kg wearable, miniaturized device with a sorbent-based hemodialysis system that is worn on the waist like a toolkit belt and is currently under development at the University of Washington, USA. An exploratory clinical trial with 10 patients received therapy with a wearable artificial kidney for 24 h [33]. However, the trial was stopped after the inclusion of the 7th subject because of device-related technical problems, including an excessive presence of carbon dioxide bubbles in the dialysate circuit, which exceeded its degassing capacity; tubing kinks; and variable pump function that resulted in fluctuating blood and dialysate flow rates. Other technical complications observed were clotting, hemolysis, subdialysis due to ammonia saturation of the sorbent column, gas bubbles in the circuit, and problems with the battery. Five subjects noted to have premature ventricular contractions while monitoring on continuous telemetry. In addition, the long-term safety of such devices has not been established yet.
Finally, other potential innovations in these bioengineering kidneys would be the incorporation of nanotechnology, innovations in battery design, and new adsorbent materials that are able to adsorb urea, avoiding the need of urease activity, thus substantially reducing carbon dioxide gas production in the dialysate circuit.
Potential AI and ML Applications for Chronic Hemodialysis Patients
New possibilities will surely arise from the applications of AI and ML to dialysis machines, although, currently, there are hardly any reported previous experiences [34]. What types of AI and ML applications are emerging for dialysis? The majority of them are based on patient monitoring, prediction models, or on medical image analysis. A pilot program using e-technologies to detect chronic kidney disease (CKD) was conducted in Australia (Electronic Diagnosis and Management Assistance to Primary Care in Chronic Kidney Disease; EMAP-CKD). The software was built on algorithms trained to identify at-risk patients and to order a relevant screening test for CKD [35]. A digital messaging platform, EpxDialysis, has improved patient-to-dialysis center communication and adherence to dialysis therapy via text messaging and telephone technology [36]. ML has also been used to develop a device to improve dialysis treatment and optimize the shape of an arteriovenous fistula, and to suppress blood flow turbulences in preclinical trials [37]. In addition, AI application tools are being used to manage anemia in routine clinical practice in dialysis patients applying an algorithm that uses a data-driven computational intelligence model based on an ANN architecture (the anemia control model trial; ANEMEX trial) to recommend individualized medication dosages based on demographics and dosage history (ClinicalTrials.gov Identifier: NCT03214627). The ANEMEX trial is estimated to finish in March 2020, and, if successful, it will help to prove the validity of AI application tools for managing CKD. An expert system reproducing human analysis for continuous monitoring of the ionic dialysance with the aim to help medical doctors to focus on the abnormal cases has also been tested [38]. The sensitivity of the automated analysis was found to be 92%, with a corresponding specificity of 75%.
Another industrial system capable of improving the quality of life of patients based on the learning of patients' health trends and their analysis to identify or predict unusual changes is known as LYTICS (https://lytics.ai/). The company's suit of solutions is based on a Big Data approach and includes applications for making predictions about hospital admissions and readmission rates, as well as a patient monitoring system that alerts clinicians when the status of a patient changes unexpectedly. The electronic data capture system LYTICS SIF is initially used to collect data from patients and is later encoded by the encryption system LYTICS SYN and sent to a database to which AI techniques are applied (LYTICS EIR, LYTICS LIN, and LYTICS VÖR) to find patterns and make predictions. LYTICS EIR is a clinical DSS designed for individualized therapies, and LYTIC VÖR is used for the continuous detection and finding of foreseen and unforeseen patterns in patients' data. Wearable and implantable technology is worn by the patient to monitor vital signals, and information is continuously relayed to computer monitors. Alerts are also sent via text message directly to members of the health-care team. It is not clear from the company's website if the LYTICS solutions are cloud based and include a mobile app component as opposed to the data being stored on local hospital computers [39].
Robotics are also steadily being introduced in health care, especially in surgery, with systems such as the da Vinci® Surgical System that involves a magnified 3D high-definition computer vision system (part of it aided by ML) and tiny wristed instruments that bend and rotate more complexly than the human hand (http://www.davincisurgery.com/). An autonomous image-guided robotic needle insertion for blood draws and intravenous insertions has also been designed that combines robotics, AI, computer vision, and image technology (http://www.veebot.com/). It must be made clear that a dialysis machine is not a AI-guided robot, since it is not able to respond to its environments in ways that humans have not explicitly taught it to [40]. However, it is easy to imagine future dialysis robots capable of carrying out complex series of actions automatically or in a semi-autonomous fashion.
Finally, it should be noted that big tech firms have quickly started to heavily invest in health care as a strategic objective. Microsoft has launched the Healthcare NExT initiative with the aim to use the cloud and AI research and industry to transform current health care (https://www.microsoft.com/en-us/microsoft-health). In addition, Google, restructured into Alphabet in 2015 to gather a group of 7 companies (https://abc.xyz/), some of them focused on health through firms such as DeepMind, focused on AI research (https://deepmind.com/), Life Science (works on the glucose-sensing contact lens), and Verily that is developing tools for individualized patients care management and is also focused on precision medicine (https://verily.com/). Other tech firms such as Apple have also taken a step towards the analysis of electronic health records, allowing patients to aggregate their records on their iPhones (https://www.apple.com/healthcare/), and the next is likely to be Facebook [41]. However, for AI-based tech firms focused on precision and personalized medicine, kidney diseases and the development of new wearable and safe dialysis devices are not yet part of their acknowledged priorities.
Conclusions
Recent advances in the fields of artificial organs and regenerative medicine are joining forces in the areas of organ transplantation and bioengineering to solve continued challenges for dialysis patients. AI, ML, and robotics will improve dialysis therapy. However, much innovation in AI and ML is still needed before the ultimate goal of the design of an operative smart dialysis machine, able to analyze and understand changes in patient homeostasis and respond appropriately in real time, is achieved. Prescription of operational parameters and delivery data displays should be easily accessible via a customized easy software interface. Currently, dialysis must be operated by nephrologists to provide patients with an accurate functional program, as well as with the delivery of the scheduled therapy. Furthermore, there are still major concerns about safety, since deep learning, the currently most promising ML approach, can still be considered a kind of “black box,” mostly unable to explain its inner workings. These techniques, when faced with life-and-death decision-making, leave human experts without a firm understanding of how the machine arrives to its recommendation, even if those recommendations may have shown to be correct in the past.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
Palex, Medtronic and Baxter provided funds for the celebration of the “2nd Meeting of Science and Dialysis.”
Author Contributions
The contributions of each author are as follows: M.H. conceived and drafted the manuscript; E.N. helped drafting the manuscript; D.S. helped drafting the manuscript; J.M.C. provided intellectual content of critical importance to the work.
Acknowledgment
We acknowledge our invited speakers at the “2nd Meeting of Science and Dialysis” for their generous support of this initiative; medical doctors, nurses, and patients of the Dialysis Units for their interest in the technology and the future of dialysis devices; the Direction of Hospital Universitari de Bellvitge for their technical assistance in the organization of the meeting, as well as Rosa Perez-Garzón for her excellent administrative work. We thank CERCA Program/Generalitat de Catalunya for institutional support.
References
- 1.Sharma K, Rupprecht C, Caroli A, Aparicio MC, Remuzzi A, Baust M, et al. Automatic Segmentation of Kidneys using Deep Learning for Total Kidney Volume Quantification in Autosomal Dominant Polycystic Kidney Disease. Sci Rep. 2017 May;7((1)):2049. doi: 10.1038/s41598-017-01779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akl AI, Sobh MA, Enab YM, Tattersall J. Artificial intelligence: a new approach for prescription and monitoring of hemodialysis therapy. Am J Kidney Dis. 2001 Dec;38((6)):1277–83. doi: 10.1053/ajkd.2001.29225. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri C, Molina M, Ponce P, Tothova M, Cattinelli I, Ion Titapiccolo J, et al. An international observational study suggests that artificial intelligence for clinical decision support optimizes anemia management in hemodialysis patients. Kidney Int. 2016 Aug;90((2)):422–9. doi: 10.1016/j.kint.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 4.Choi JW, Lee H, Lee JC, Lee S, Kim YS, Yoon HJ, et al. Application of genetic algorithm for hemodialysis schedule optimization. Comput Methods Programs Biomed. 2017 Jul;145:35–43. doi: 10.1016/j.cmpb.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 5. Chris O'Connor: Managing Engineering Complexity: Are you ready? Available from: https://www.ibm.com/blog/internet-of-things/iot-managing-engeenering-complexity/
- 6.Waqaas Al-Siddiq The impact of Artificial Intelligence on Medtech. Available from: https://www.mpo-mag.com/contents/view_online-exclusives/2017-04-07/the-impact-of-artificial-intelligence-on-medtec.
- 7.Santoro A, Mancini E, Basile C, Amoroso L, Di Giulio S, Usberti M, et al. Blood volume controlled hemodialysis in hypotension-prone patients: a randomized, multicenter controlled trial. Kidney Int. 2002 Sep;62((3)):1034–45. doi: 10.1046/j.1523-1755.2002.00511.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark WR, Neri M, Garzotto F, Ricci Z, Goldstein SL, Ding X, et al. The future of critical care: renal support in 2027. Crit Care. 2017 Apr;21((1)):92. doi: 10.1186/s13054-017-1665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Chakravarthi MR, Ricci Z, Tolwani A, Neri M, De Rosa S, et al. ADQI Consensus Group Precision Continuous Renal Replacement Therapy and Solute Control. Blood Purif. 2016;42((3)):238–47. doi: 10.1159/000448507. [DOI] [PubMed] [Google Scholar]
- 10.Cerdá J, Baldwin I, Honore PM, Villa G, Kellum JA, Ronco C, ADQI Consensus Group Role of Technology for the Management of AKI in Critically Ill Patients: From Adoptive Technology to Precision Continuous Renal Replacement Therapy. Blood Purif. 2016;42((3)):248–65. doi: 10.1159/000448527. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015 Jun;385((9987)):2616–43. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 12.Li P, Qu LP, Qi D, Shen B, Wang YM, Xu JR, et al. High-dose versus low-dose haemofiltration for the treatment of critically ill patients with acute kidney injury: an updated systematic review and meta-analysis. BMJ Open. 2017 Oct;7((10)):e014171. doi: 10.1136/bmjopen-2016-014171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol. 2014 Sep;9((9)):1627–38. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humes HD, Buffington D, Westover AJ, Roy S, Fissell WH. The bioartificial kidney: current status and future promise. Pediatr Nephrol. 2014 Mar;29((3)):343–51. doi: 10.1007/s00467-013-2467-y. [DOI] [PubMed] [Google Scholar]
- 15.Song JH, Humes HD. The bioartificial kidney in the treatment of acute kidney injury. Curr Drug Targets. 2009 Dec;10((12)):1227–34. doi: 10.2174/138945009789753273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humes HD, Sobota JT, Ding F, Song JH, Group RI, RAD Investigator Group A selective cytopheretic inhibitory device to treat the immunological dysregulation of acute and chronic renal failure. Blood Purif. 2010;29((2)):183–90. doi: 10.1159/000245645. [DOI] [PubMed] [Google Scholar]
- 17.Pino CJ, Yevzlin AS, Tumlin J, Humes HD. Cell-based strategies for the treatment of kidney dysfunction: a review. Blood Purif. 2012;34((2)):117–23. doi: 10.1159/000341649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brian Blum Saving Lives in the ICU through artificial intelligence. Available from: https://www.Israel 21c.org. [Google Scholar]
- 19.Davoudi A, Malhotra KR, Shickel B, Siegel S, Williams S, Ruppert M, et al. The Intelligent ICU Pilot Study: Using Artificial Intelligence Technology for Autonomous Patient Monitoring. arXiv:1804.10201. 2018 [Google Scholar]
- 20.Powles J, Hodson H. Google DeepMind and healthcare in an age of algorithms. Health Technol (Berl) 2017;7((4)):351–67. doi: 10.1007/s12553-017-0179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah H. The DeepMind debacle demands dialogue on data. Nature. 2017 Jul;547((7663)):259. doi: 10.1038/547259a. [DOI] [PubMed] [Google Scholar]
- 22.Wing JM. Computational thinking and thinking about computing. Philos Trans A Math Phys. Eng Sci. 1881;2008((366)):3717–25. doi: 10.1098/rsta.2008.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laura Pappano Learning to think like a computer. The New York Times. April 4, 2017 Available from: https://www.nytimes.com/2017/04/04/education/edlife/teaching-students-computer-code.html. [Google Scholar]
- 24.Chung HC, Ko IK, Atala A, Yoo JJ. Cell-based therapy for kidney disease. Korean J Urol. 2015 Jun;56((6)):412–21. doi: 10.4111/kju.2015.56.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Araoka T, Wu J, Liao HK, Li M, Lazo M, et al. 3D Culture Supports Long-Term Expansion of Mouse and Human Nephrogenic Progenitors. Cell Stem Cell. 2016 Oct;19((4)):516–29. doi: 10.1016/j.stem.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bantounas I, Ranjzad P, Tengku F, Silajdžić E, Forster D, Asselin MC, et al. Generation of Functioning Nephrons by Implanting Human Pluripotent Stem Cell-Derived Kidney Progenitors. Stem Cell Reports. 2018 Mar;10((3)):766–79. doi: 10.1016/j.stemcr.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montserrat N, Garreta E, Izpisua Belmonte JC. Regenerative strategies for kidney engineering. FEBS J. 2016 Sep;283((18)):3303–24. doi: 10.1111/febs.13704. [DOI] [PubMed] [Google Scholar]
- 28.Orlando G, Farney AC, Iskandar SS, Mirmalek-Sani SH, Sullivan DC, Moran E, et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg. 2012 Aug;256((2)):363–70. doi: 10.1097/SLA.0b013e31825a02ab. [DOI] [PubMed] [Google Scholar]
- 29.Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact Mater. 2018 Feb;3((2)):144–56. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Fissell WH, Humes DH, Roy S. Current strategies and challenges in engineering a bioartificial kidney. Front Biosci (Elite Ed) 2015 Jan;7((2)):215–28. doi: 10.2741/e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kensinger C, Karp S, Kant R, Chui BW, Goldman K, Yeager T, et al. First Implantation of Silicon Nanopore Membrane Hemofilters. ASAIO J. 2016 Jul-Aug;62((4)):491–5. doi: 10.1097/MAT.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buck AK, Goebel SG, Goodin MS, Wright NJ, Groszek JJ, Moyer J, et al. Original article submission: platelet stress accumulation analysis to predict thrombogenicity of an artificial kidney. J Biomech. 2018 Mar;69:26–33. doi: 10.1016/j.jbiomech.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gura V, Rivara MB, Bieber S, Munshi R, Smith NC, Linke L, et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight. 2016 Jun;1((8)):e86397. doi: 10.1172/jci.insight.86397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hueso M, Vellido A, Montero N, Barbieri C, Ramos R, Angoso M, et al. Artificial Intelligence for the Artificial Kidney: Pointers to the Future of a Personalized Hemodialysis Therapy. Kidney Dis (Basel) 2018 Feb;4((1)):1–9. doi: 10.1159/000486394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venuthurupalli SK, Hoy WE, Healy HG, Cameron A, Fassett RG. CKD Screening and Surveillance in Australia: Past, Present, and Future. Kidney Int Rep. 2017 Oct;3((1)):36–46. doi: 10.1016/j.ekir.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Som A, Groenendyk J, An T, Patel K, Peters R, Polites G, et al. Improving Dialysis Adherence for High Risk Patients Using Automated Messaging: proof of Concept. Sci Rep. 2017 Jun;7((1)):4177. doi: 10.1038/s41598-017-03184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iori F, Grechy L, Corbett RW, Gedroyc W, Duncan N, Caro CG, Vincent PE. The effect of in-plane arterial curvature on blood flow and oxygen transport in arterio-venous fistulae. Phys Fluids (1994) 2015;27((3)):031903. doi: 10.1063/1.4913754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chanliau J, Charasse C, Rose C, Béné B. Clinical evaluation of an expert system for arteriovenous fistula assessment. Int J Artif Organs. 2014 Nov;37((11)):809–15. doi: 10.5301/ijao.5000364. [DOI] [PubMed] [Google Scholar]
- 39. https://lytics.ai/solution/end-stage-renal-disease-esrd-and-esco.
- 40.Matt Simon What is a Robot? Available from: https://www.wired.com/story/what-is-a-robot/)
- 41.Rachel Z, Arndt Is Facebook making moves in healthcare? Available from: http://www.modernhealthcare.com/article/20180427/NEWS/180429912.