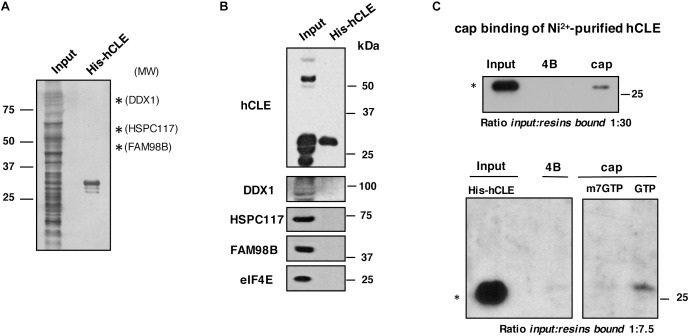
FIGURE 4.
Purified hCLE binds cap analog. (A) Silver staining of Ni2+-purified His-hCLE protein. Only hCLE is visible after purification; arrows indicate molecular weight for associated proteins. (B) Western blot analysis of indicated proteins in total extracts (Input) or purified fraction of His-hCLE. To obtain the most accurate results, we analyzed all the proteins from the same sample and experiment. (C) (Top); Binding of Ni2+-purified His-hCLE proteins to cap analog and control resins. Bound protein was analyzed by Western blot. The relative amounts applied to SDS-gels were Input:resins bound 1:30. (Bottom); Purified His-hCLE was incubated with cap analog (cap) or control resins (4B) in the presence of 10 mM of GTP, or m7GTP as competitors and His-hCLE binding was analyzed by Western blot. The relative amounts applied to SDS-gels were Input:resins bound 1:7.5. The experiments were repeated at least three times. ∗monomer.