Table 2.
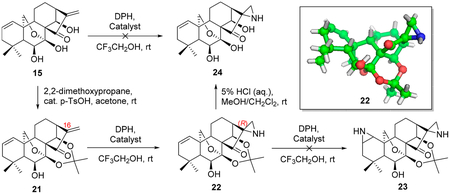
Exploration and Optimization of Oridonin-Based Aziridinationa
| entries | DPH amount (equiv) | catalystb | temp/time | recovery of 21c | yield of productsc |
|---|---|---|---|---|---|
| 1 | 2.2 | Rh2(esp)2 | rt./12 h | trace | 22 (69%) |
| 2 | 2.2 | Rh2(OAc)4 | rt./12 h | trace | 22 (58%) |
| 3 | 2.2 | Rh2(TPA)4 | rt./12 h | >80% | 22 (trace) |
| 4 | 2.2 | Rh2(R-DOSP)4 | rt./12 h | >80% | 22 (NA)d |
| 5 | 2.2 | Rh2(S-DOSP)4 | rt./12 h | >80% | 22 (NA) |
| 6 | 1.2 | Rh2(esp)2 | rt./12 h | 18% | 22 (54%) |
| 7 | 1.5 | Rh2(esp)2 | rt./12 h | 5% | 22 (66%) |
| 8 | 4.4 | Rh2(esp)2 | rt./24 h | NA | 22 (71%) |
| 23 (NA) | |||||
| 9 | 4.4 | Rh2(esp)2 | 50°C/24 h | NA | 22 (66%) |
| 23 (NA) | |||||
| 10 | 4.4 | Rh2(esp)2 | reflux/48 h | NA | 22 (56%) |
| 23 (NA) |
All reaction conditions in this table were performed, utilizing 21 as the substrate.
The equivalent of catalyst is 5%.
The data were based on isolated yields.
NA: not available.
