Abstract
Background/purpose
Denture soft liners, especially used for elders who have poor disinfection habits, provide a favourable environment for accumulation and colonization of microorganisms. This in vitro study is aimed to investigate the effectiveness of natural carvacrol incorporation into soft lining material on the inhibition of oral pathogens.
Materials and methods
Antimicrobial susceptibility of carvacrol was primarily determined by disc diffusion method. Soft lining material was prepared as recommended by the manufacturer and 10 μL carvacrol was added aseptically to the soft liner discs. Inhibition zones for the control discs without carvacrol (C) and carvacrol-incorporated discs (CL) were determined by disc diffusion method. The biofilm inhibition percentages of carvacrol on soft liner was determined by MTT assay and also observed by Scanning Electron Microscopy (SEM).
Results
Carvacrol displayed great antimicrobial activity for yeast, Gram-negative and Gram-positive strains. The highest inhibition zone of carvacrol (41.33 ± 1.53 mm) was measured for Bacillus subtilis strain which is followed by Candida albicans and Streptococcus sanguis (34.00 ± 1.73 mm and 32.33 ± 0.58 mm, respectively). The inhibition zones were also similar for soft liner discs with carvacrol, with the highest inhibition zones against B. subtilis, Streptococcus mutans and C. albicans (43.67 ± 0.58 mm, 40.33 ± 0.58 mm and 38.33 ± 1.15 mm, respectively). Incorporation of carvacrol into the soft liner decreased (98.03 ± 0.2%) of the biofilm formation for C. albicans.
Conclusion
Carvacrol-incorporation obviously decreased the colonization and plaque formation of oral pathogens, especially C. albicans accumulation. Carvacrol may be useful as a promising agent for antibacterial and antifungal management for denture soft lining materials.
Keywords: antibacterial, antibiofilm, antifungal, carvacrol, soft liner
Introduction
The oral cavity contains numerous species of microorganisms which include bacteria, viruses, and fungi organized into dental plaque. These microorganisms are able to form biofilms, which are resistant to mechanical stress or antibiotic treatment.1 Dental plaque is a film of microorganisms on the tooth surface that plays an important part in the development of caries and periodontal diseases.2 Patients who use removable prosthetic devices may have oral health problems if they do not apply adequate hygiene rules. The most common lession is denture stomatitis which is characterized as inflammation and erythema of the oral mucosal areas covered by the denture.3, 4, 5 There are many reports suggest that most of the patients who wear removable dentures can suffer from denture stomatitis.5, 6, 7, 8 Denture relining materials are permanently used for sensitive patients to limit the traumatic effect of dentures by providing cushioning effect and also for denture relining to evenly distribute the loads transferred onto soft tissues during motion.9, 10 They are primarily used in patients with thin atrophic mucosa, with normal mucosa with an atrophied ridge, with a sharp alveolar ridge and when the mucosa exhibits a low tolerance to the load applied by the dentures.11, 12
Soft lining materials are easily contaminated in the oral environment and it is not possible to clean or brush effectively.13 For creating an efficient denture disinfection, the hygiene of the denture soft lining materials or tissue conditioners play significant role.10 Denture disinfectant agents such as chlorhexidine gluconate, sodium hypochlorite, hydrogen peroxide and more were reported to cause unfavourable changes to the physical and chemical properties of the soft liner.14, 15 Studies have reported that the fungi and bacterial species can enter porous spaces within the denture liner and that their colonization may reduce the intra-oral life of the material.9, 16 Antibiofilm activity of the soft liner is also an efficient feature to prevent the patients from infections with resident oral microorganisms.17 It is essential to apply the best infection management strategy for the risk group of denture wearers.13 Douglas and Walker18 had the idea of enhancing the therapeutic effects of a tissue conditioner with an antifungal agent to prolong the action of drug and treat the tissue trauma with lower cost. An antimicrobial soft liner will help to patients who can not perform routine denture care. Medicinal herbs are nowadays using as an alternative treatment method as a naturopathic remedy.19
Carvacrol (2-methyl-5-(1-methylethyl) phenol) (C10 H14O) is an essential oil component of numerous aromatic plants such as oregano and thyme (Thymus and Origanum sp.).20, 21, 22 It has been granted Generally Recognized as Safe (GRAS) status by the FDA in 21 CFR (Code of Federal Regulation) part 172.515.23 Antimicrobial activity of carvacrol has been demonstrated against bacteria, mold and yeast.22, 24 The inhibitory effect of its volatile phase against bacteria was also studied.25 Recently some antimicrobial drugs, such as eugenol, have the most attention for dental cleaning purpose. As far as we know, there is no study about the use of carvacrol for denture soft lining applications. With this current study, it is aimed to investigate the antimicrobial properties of a soft liner incorporated with a natural agent which posses great biocidal activity against several microorganisms. For this purpose, antimicrobial and antibiofilm activities of carvacrol and carvacrol-incorporated soft liner have been studied.
Material and methods
Materials
Carvacrol was purchased from Sigma–Aldrich. Sabouraud Dextrose Broth/Agar, Brain Heart Infusion Broth/Agar, Mueller Hinton Agar, Tryptic Soya Broth and d-Glucose were purchased from Merck. Autopolymerized soft denture liner material (UFI Gel) is based on A-silicone (addition silicone) and consists from mixture of different polyalkylsiloxanes, fumed silica, catalysts, butanone and additives. UFI Gel P (Voco GMBH, Cuxhaven, Germany) were purchased from a local dental market. Test microorganisms were all provided from Mugla Sitki Kocman University Culture Collection.
Microbial strains and culture conditions
The antimicrobial activity of the carvacrol was individually tested to a group of microorganisms including; Candida albicans ATCC 10239 (yeast) which is directly associated with the pathogenesis of denture stomatitis; Staphylococcus aureus ATCC 25923 (Gram/+), Streptococcus mutans ATCC 25175 (Gram/+) and Streptococcus sanguis ATCC 10556 (Gram/+) which are known to be oral pathogens; Bacillus subtilis ATCC 6633 (Gram/+), Escherichia coli ATCC 25922 (Gram/-) and Pseudomonas aeruginosa ATCC 27853 (Gram/−) which are standard pathogenic strains for antimicrobial studies and represent the clinically important strains of Gram (+) and Gram (−) bacteria. All strains were provided from Culture Collection of Mugla Sitki Kocman University (MUKK). B. subtilis, E. coli and S. aureus strains were incubated at 37 ± 0.1 °C for 24–48 h, C. albicans and P. aeruginosa were incubated at 30 ± 0.1 °C for 24–48 h and Streptococcus strains were incubated anaerobically at 37 ± 0.1 °C for 48–72 h. Inocula were prepared adjusting the turbidity of the medium to match the 0.5 McFarland standard dilutions. The strains were maintained in their appropriate agar slants at 4 °C throughout the study and used as stock cultures.
Disc diffusion assay of carvacrol
The antimicrobial activity was measured based on the disc diffusion method.26 Each microbial suspension (0.1 mL) was spreaded on agar plates. Sterile 6 mm paper discs (Schleicher and Schuell) were impregnated with 10 μL of essential oil. The discs were allowed to dry and were then placed on the inoculated agar. The plates were incubated at appropriate temperature for performing the microorganisms, as mentioned above. At the end of the incubation period, diameters of growth zones around the disks were measured. The experiments were replicated for three times.
Disc diffusion assay of soft liner incorporated with carvacrol
UFI Gel P soft liner was prepared according to the manufacturer's recommendation with modification by addition of 10 μL carvacrol.27, 28 Before deciding the amount of the carvacrol, a pilot study was employed. Different concentrations of carvacrol has been tried (0.5, 1, 2.5, 5, 10, 20 and 50 μL) and 10 μL of carvacrol was found to be the most effective concentration to exhibit the antimicrobial and antibiofilm activities. Also, on the other hand, this concentration was the lowest amount which did not alter the physical properties of the soft liner, such as colour and homogenity. Sterile circular thin discs with 5 mm diameter and 1 mm thickness were finally prepared using a sterile cutter. Briefly; for preparing the soft liner, adhesive and catalyst (1:1) dispensed from the tubes (equal length and equal weight) and mixed for 30 s to form a homogenous and bubble-free consistency. Just before the mixing process, carvacrol was added (10 μL for each 5 mm soft liner disc) and mixed together with the adhesive and catalyst. Similar drug-free soft liner discs were also prepared as control (negative control). Carvacrol containing group (CL) and control group (C) discs were aseptically stored. Before the experiments, both sides of the discs were treated with UV application for 2 min. Antimicrobial activity of carvacrol-incorporated soft liner was determined by disc diffusion method. Each microbial suspension (0.1 mL) was spreaded on agar plates. Sterilized soft liner discs (CL and C groups) were then placed on the inoculated agar. The plates were incubated at appropriate temperature for performing the microorganisms, as mentioned above. At the end of the incubation period, zone of inhibition was determined by measuring the diameter of microbial clearance. The experiments were replicated for three times.
Evaluating the antibiofilm activity
Antibiofilm activity of the soft liner incorporated with carvacrol was measured by MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide staining.29 Briefly, soft liner discs were incubated with microbial inoculums in Tryptone soy broth-TSB (supplemented with 5% d-Glucose) for 72 h. After incubation, the media was poured and the discs were washed with PBS. The discs were sonicated in 5 mL PBS for 5 min to remove the attached microbial biofilm. The discs were removed and the solution was centrifugated. The pellet was suspended with 150 μL PBS in microplate wells. 50 μL MTT solution added to the wells for staining live microorganisms and incubated for 2 h at 37 °C. After removing the medium from the wells, 150 μL DMSO and 25 μL glicin buffer were added. The absorbances were read at 550 nm in microplate reader (Multiskan GO UV/Vis Microplate Spectrophotometer, Thermo-Fisher Scientific, USA). Results compared with the control discs that were not containing carvacrol and percentage of biofilm inhibition was calculated.
Evaluating the antibiofilm activity by scanning electron microscopy
The inhibition effect of carvacrol on biofilm formation occur on the soft liner was visualized by Scanning Electron Microscope (JSM 7600-F, Jeol, Japan). For this purpose, microbial strains were activated at Tryptone soy broth-TSB (supplemented with 5% d-Glucose) and grown up to stationary phase. Fresh inocula were prepared adjusting the turbidity of the medium to match the 0.5 McFarland standard dilutions. Then sterilized soft liner discs (CL and C groups) were put in each microbial suspension and incubated at appropriate temperatures, as mentioned above. At the end of the incubation period, discs were removed and washed with PBS (0.1 M, pH 7.4) for two times. Discs were fixed in glutaraldehyde and dehydrated in ascending ethanol solutions ranging from 70 to 100%. The discs were then sputter coated with gold using sputter coater (Emitech K550X, Emitech Ltd, Ashford, UK) and imaged via SEM with an accelerating voltage of 15 keV.
Results
Disc diffusion analysis of carvacrol exhibited significant antimicrobial activity against the tested microorganism (Table 1). The highest inhibition zone of 41.33 ± 1.53 mm was measured for B. subtilis.
Table 1.
Antimicrobial activity of carvacrol by disc diffusion method.
| Microroganism | Inhibition zone (mm) |
||
|---|---|---|---|
| Antibiotica | Control | Carvacrol discsb | |
| Candida albicans | 24 | — | 34.00 ± 1.73 |
| Bacillus subtilis | 19 | — | 41.33 ± 1.53 |
| Escherichia coli | 23 | — | 25.33 ± 0.58 |
| Pseudomonas aeruginosa | 21 | — | 16.67 ± 1.53 |
| Staphylococcus aureus | 20 | — | 24.67 ± 1.53 |
| Streptococcus mutans | 31 | — | 28.67 ± 1.15 |
| Streptococcus sanguis | 29 | — | 32.33 ± 0.58 |
(−): No activity.
Standard antibiotic discs used for microorganism are: C.albicans, nystatin (30 μg); B. subtilis, amoxicillin + clavulanic acid (20 μg/10 μg); S. aureus, S. mutans and S. sanguis ampicilin (10 μg); E.coli, cefoperazone (75 μg); P. aeruginosa, gentamicin (10 μg).
Values are presented as mean ± standard deviation (SD).
Standard soft liner discs without carvacrol (control) showed no inhibition zone for all tested microorganism. Carvacrol-incorporation into soft liner (CL group) resulted in great antimicrobial efficiency against all tested microorganisms (Table 2).
Table 2.
Antimicrobial activity of soft liner incorporated with carvacrol.
| Microroganism | Inhibition zone (mm) |
|
|---|---|---|
| Control | CLa | |
| Candida albicans | — | 38.33 ± 1.15 |
| Bacillus subtilis | — | 43.67 ± 0.58 |
| Escherichia coli | — | 29.33 ± 1.15 |
| Pseudomonas aeruginosa | — | 15.33 ± 0.58 |
| Staphylococcus aureus | — | 26.67 ± 1.53 |
| Streptococcus mutans | — | 40.33 ± 0.58 |
| Streptococcus sanguis | — | 36.67 ± 1.15 |
(−): No activity.
Values are presented as mean ± standard deviation (SD).
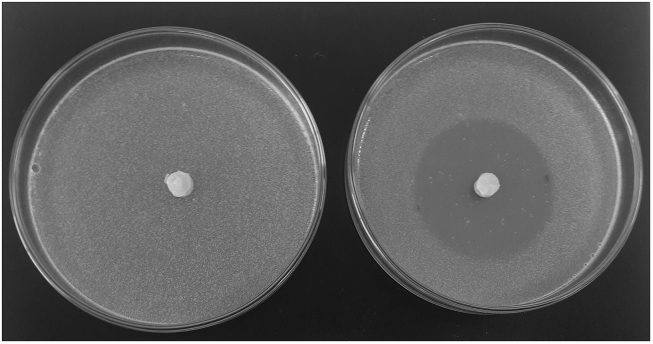
Similar to carvacrol impregnated discs, the highest inhibition zone was also obtained for B. subtilis (43.67 ± 0.58 mm) (Fig. 1).
Figure 1.
The highest inhibition zone obtained for B. subtilis via disc diffusion assay.
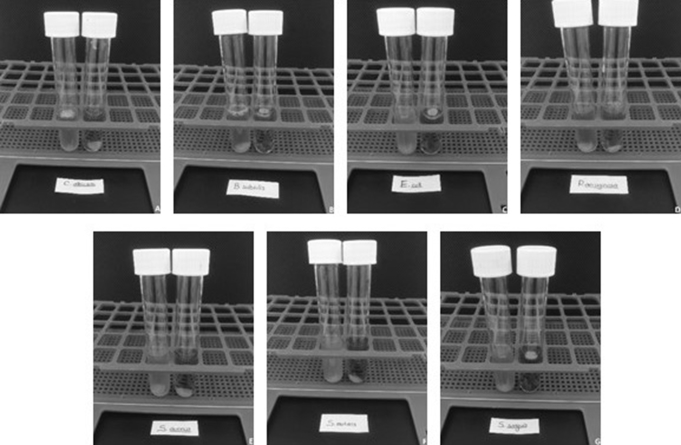
In order to determine the inhibition effect of carvacrol-incorporation on the biofilm formation onto the soft liner discs, MTT staining was employed (Fig. 2). Visually, all carvacrol-incorporated discs were clear than the control group which confirms the biofilm inhibition.
Figure 2.
Tube images of control and carvacrol-incorporated soft liner incubated for biofilm formation. A, C. albicans; B, B. subtilis; C, E. coli; D, P. aeruginosa; E, S. aureus; F, S. mutans and G, S. sanguis.
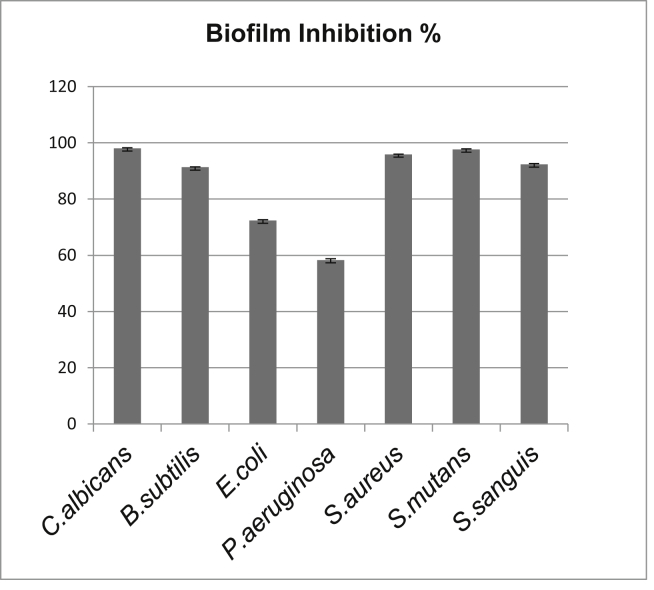
When comparing with the control group, biofilm inhibition percentages of carvacrol-incorporated soft liners are given in Fig. 3. The highest inhibition rate was observed against C. albicans biofilm formation (98.03 ± 0.2%). All tested microorganisms were found to be sensitive against carvacrol-incorporation. Results were similar with the antimicrobial assay. Decrease of P. aeruginosa biofilm formation by carvacrol-incorporated soft liner is measured as 58.33 ± 0.4%.
Figure 3.
Antibiofilm activity of soft liner incorporated with carvacrol presented as % inhibition.
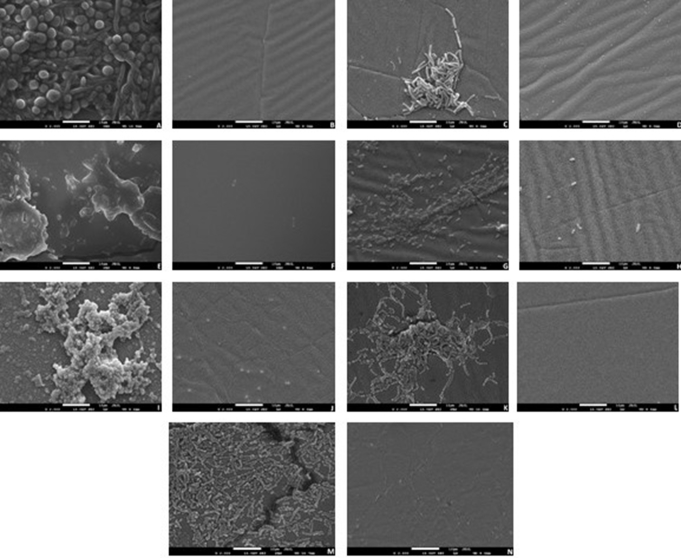
Biofilm inhibition on the carvacrol-incorporated soft liner was also monitored by SEM (Fig. 4). The biofilm formation on the control discs which did not contain carvacrol were highly intense for all tested microorganism. Candidal biofilm was the most compact formation when comparing with the bacterial strains. P. aeruginosa and E. coli biofilms were found to have thin biofilm matrix while the Gram-positive bacteria cells colonized and accumulated into the biofilm matrix. It was clear that adding carvacrol in the soft lining denture material itself prevent the microbial adhesion. These findings are the observable proof of the antimicrobial and antibiofilm activity of carvacrol addition to the soft liner.
Figure 4.
SEM images of biofilm inhibition on the surface of the soft liner incorporated with carvacrol. All images are taken at ×2000 magnification. C. albicans (A: Control, B: Carvacrol treatment); B. subtilis (C: Control, D: Carvacrol treatment); E. coli (E: Control, F: Carvacrol treatment); P. aeruginosa (G: Control, H: Carvacrol treatment); S. aureus (I: Control, J: Carvacrol treatment); S. mutans (K: Control, L: Carvacrol treatment); S. sanguis (M: Control, N: Carvacrol treatment).
Discussion
Denture hygiene is directly related to the denture plaque and biofilm formation. Plaque and biofilm layer contain several microorganisms including bacteria and yeast.30 Soft lining denture material is a predisposing area for microbial accumulation as it is placed in oral cavity and related to the tissues with inflammation.28 Natural antimicrobial compounds have potential to inhibit the growth of microorganism in the biofilm formation and promise as an alternative therapeutic agent. Common treatment methods that are using antifungal drugs may have limited action for penetrating and reacting into the biofilm matrix.31 Medicinal plants are of great interest for designing potentially active biomaterials. This present study incorporated carvacrol into soft liner and its antimicrobial antibiofilm activities against growth of pathogenic microorganisms were evaluated.
Çetin et al.32 (2011) studied the antimicrobial effects of the essential oils from oregano (Origanum acutidens and Origanum rotundifolium) and thyme (Thymus sipyleus subsp. sipyleus var. rosulans) all of which have carvacrol as the major chemical compound. They obtained the highest inhibition zone (72 mm) for O. acutidens essential oil (10 μL) against B. subtilis. All inhibition zones of carvacrol-impregnated discs measured in this study were higher than the zone in diameter around the standard antibiotic discs. C. albicans was also found to be sensitive against carvacrol with the inhibition zone of 34 mm. Ultee et al.33 (2002) reported that the presence of the phenolic hydroxyl group of the carvacrol is the important key for its antimicrobial activity against food-borne pathogen Bacillus cereus.
There are studies about incorporating antifungal agents to resilient liners such as nystatin.29, 34 Pachava et al.19 (2015) reported that tea tree oil incorporation to denture soft liner decreased C. albicans growth significantly. Similarly, Amornvit et al.28 (2014) combined lemongrass essential oil to tissue conditioner and demonstrated the anti-Candida activity.
Dalleau et al.35 (2008) reported that carvacrol inhibited Candida biofilm by ≥ 75% when used at 0.03% concentration. Lima et al.36 (2013) suggested that carvacrol is able to act by altering the membrane structure of the fungal cell. There are some other reports about that carvacrol interacts with the cell membrane and changes its permeability.37, 38
Soft lining denture materials combined with carvacrol has shown great in vitro antimicrobial activity against microorganism including oral pathogens. Adherence and accumulation of microorganisms, especially Candida, is the most important factor in traumatic infections for denture wearers. Carvacrol-incorporation significantly reduced the biofilm formation and colonization of C. albicans on the soft liner significantly. It can be suggested that naturopathic enhancement of denture soft liners with carvacrol may be a promising solution for oral hygiene of denture wearers. This combination may be effectively used for older people who have poor disinfection habits. According to the hopefull results of the present study, required in vitro and in vivo studies will be designed to clinical use of carvacrol-incorporated denture soft-liner.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
References
- 1.Avila M., Ojcius D.M., Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405–411. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh P.D. Microbiological aspects of the chemical control of plaque and gingivitis. J Dent Res. 1992;71:1431–1438. doi: 10.1177/00220345920710071501. [DOI] [PubMed] [Google Scholar]
- 3.Arendorf T.M., Walker D.M. Denture stomatitis: a review. J Oral Rehabil. 1987;14:217–227. doi: 10.1111/j.1365-2842.1987.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson J. The aetiology, diagnosis and management of denture stomatitis. Br Dent J. 1998;185:380–384. doi: 10.1038/sj.bdj.4809821. [DOI] [PubMed] [Google Scholar]
- 5.Reichart P.A. Oral mucosal lesions in a representative cross-sectional study of aging Germans. Community Dent Oral Epidemol. 2000;28:390–398. doi: 10.1034/j.1600-0528.2000.028005390.x. [DOI] [PubMed] [Google Scholar]
- 6.Budtz-Jorgensen E. Clinical aspects of Candida infection in denture wearers. J Am Dent Assoc. 1978;96:474–477. doi: 10.14219/jada.archive.1978.0088. [DOI] [PubMed] [Google Scholar]
- 7.Webb B.C., Thomas C.J., Willcox M.D.P., Harty D.W.S., Knox K.W. Candida-associated denture stomatitis. Aetiology and management: a review. Part I. Factors influencing distribution of Candida species in the oral cavity. Aust Dent J. 1998;43:45–50. doi: 10.1111/j.1834-7819.1998.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramage G., Tomsett K., Wickers B.L., López-Ribot J.L., Redding S.W. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:53–59. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurth S., Hallikerimath R.B. An In-vitro evaluation of retention, colonization and penetration of commonly used denture lining materials by Candida albicans. J Clin Diagn Res. 2016;10:84–88. doi: 10.7860/JCDR/2016/20892.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chladek G., Mertas A., Barszczewska-Rybarek I. Antifungal activity of denture soft lining material modified by silver nanoparticles-a pilot study. Int J Mol Sci. 2011;12:4735–4744. doi: 10.3390/ijms12074735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulad K., Taylor R.L., Verran J., McCord J.F. Colonization and penetration of denture soft lining materials by Candida albicans. Dent Mater. 2004;20:167–175. doi: 10.1016/s0109-5641(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 12.Mack P.J. Denture soft linings: clinical indications. Aust Dent J. 1989;34:454–458. doi: 10.1111/j.1834-7819.1989.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 13.Atar-Froyman L., Sharon A., Weiss E.I. Anti-biofilm properties of wound dressing incorporating nonrelease polycationic antimicrobials. Biomaterials. 2015;46:141–148. doi: 10.1016/j.biomaterials.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 14.Huddar D.A., Hombesh M.N., Sandhyarani B., Chandu G.S., Nanjannawar G.S., Shetty R. Effect of denture cleanser on weight, surface roughness and tensile bond strength of two resilient denture liners. J Contemp Dent Pract. 2012;13:607–611. [PubMed] [Google Scholar]
- 15.Brożek R., Koczorowski R., Rogalewicz R., Voelkel A., Czarnecka B., Nicholson J.W. Effect of denture cleansers on chemical and mechanical behavior of selected soft lining materials. Dent Mater. 2011;27:281–290. doi: 10.1016/j.dental.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Wright P.S., Young K.A., Riggs P.D., Parker S., Kalachandra S. Evaluating the effect of soft lining materials on the growth of yeast. J Prosthet Dent. 1998;79:404–409. doi: 10.1016/s0022-3913(98)70153-2. [DOI] [PubMed] [Google Scholar]
- 17.Zender C.A., Mehta V., Pittman A.L., Feustel P.J., Jaber J.J. Etiologic causes of late osteocutaneous free flap failures in oral cavity cancer reconstruction. Laryngoscope. 2012;122:1474–1479. doi: 10.1002/lary.23326. [DOI] [PubMed] [Google Scholar]
- 18.Douglas W.H., Walker D.M. Nystatin in denture liners an alternative treatment of denture stomatitis. Brit Dent J. 1973;135:55–59. doi: 10.1038/sj.bdj.4803045. [DOI] [PubMed] [Google Scholar]
- 19.Pachava K.R., Nadendla L.K., Alluri L.S.C., Tahseen H., Sajja N.P. In vitro antifungal evaluation of denture soft liner incorporated with tea tree oil: a new therapeutic approach towards denture stomatitis. J Clin Diagn Res. 2015;9:62–64. doi: 10.7860/JCDR/2015/12396.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ultee A., Kets E.P.W., Smid E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 1999;65:4606–4610. doi: 10.1128/aem.65.10.4606-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vincenzi M., Stammati A., De Vincenzi A., Silano M. Constituents of aromatic plants: carvacrol. Fitoterapia. 2004;75:801–804. doi: 10.1016/j.fitote.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Guarda A., Rubilar J.F., Miltz J., Galotto M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int J Food Microbiol. 2011;146:144–150. doi: 10.1016/j.ijfoodmicro.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 23.CFR, Code of Federal Regulations. Title 21, Part 172.515 . 2009. Food additives permitted for direct addition to food for human consumption: Synthetic flavoring substances and adjuvants; pp. 56–63. [Google Scholar]
- 24.Knowles J.R., Roller S., Murray D.B., Naidu A.S. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar typhimurium. Appl Environ Microbiol. 2005;71:797–803. doi: 10.1128/AEM.71.2.797-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurek M., Moundanga S., Favier C., Galić K., Debeaufort F. Antimicrobial efficiency of carvacrol vapour related to mass partition coefficient when incorporated in chitosan based films aimed for active packaging. Food control. 2013;32:168–175. [Google Scholar]
- 26.Collins C.H., Lyne P.M., Grange J.M., editors. Microbiological Methods. 7th ed. Butterworths; London: 1995. [Google Scholar]
- 27.Catalán A., Pacheco J.G., Martínez A., Mondaca M.A. In vitro and in vivo activity of Melaleuca alternifolia mixed with tissue conditioner on Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:327–332. doi: 10.1016/j.tripleo.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Amornvit P., Choonharuangdej S., Srithavaj T. Lemongrass-incorporated tissue conditioner against Candida albicans culture. J Clin Diagn Res. 2014;8:50–52. doi: 10.7860/JCDR/2014/8378.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bueno M.G., Urban V.M., Barbério G.S. Effect of antimicrobial agents incorporated into resilient denture relines on the Candida albicans biofilm. Oral Dis. 2015;21:57–65. doi: 10.1111/odi.12207. [DOI] [PubMed] [Google Scholar]
- 30.Gendreau L., Loewy Z.G. Epidemiology and etiology of denture stomatitis. J Prosthodont. 2011;20:251–260. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 31.Kanathila H., Bhat A.M., Krishna P.D. The effectiveness of magnesium oxide combined with tissue conditioners in inhibiting the growth of Candida albicans: an in vitro study. Indian J Dent Res. 2011;22:613. doi: 10.4103/0970-9290.90324. [DOI] [PubMed] [Google Scholar]
- 32.Cetin B., Cakmakci S., Cakmakci R. The investigation of antimicrobial activity of thyme and Oregano essential oils. Turk J Agric For. 2011;35:145–154. [Google Scholar]
- 33.Ultee A., Bennik M.H.J., Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chopde N., Pharande A., Khade M.N., Khadtare Y.R., Shah S.S., Apratim A. In vitro antifungal activity of two tissue conditioners combined with nystatin, miconazole and fluconazole against Candida albicans. J Contemp Dent Pract. 2012;13:695–698. doi: 10.5005/jp-journals-10024-1211. [DOI] [PubMed] [Google Scholar]
- 35.Dalleau S., Cateau E., Bergès T., Berjeaud J.M., Imbert C. In vitro activity of terpenes against Candida biofilms. Int J Antimicrob Agents. 2008;31:572–576. doi: 10.1016/j.ijantimicag.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 36.Lima I.O., Pereira F.D.O., Oliveira W.A.D. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J Essent Oil Res. 2013;25:138–142. [Google Scholar]
- 37.Helander I.M., Alakomi H.L., Latva-Kala K. Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem. 1998;46:3590–3595. [Google Scholar]
- 38.Lambert R.J.W., Skandamis P.N., Coote P.J., Nychas G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J App Microbiol. 2001;91:453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]