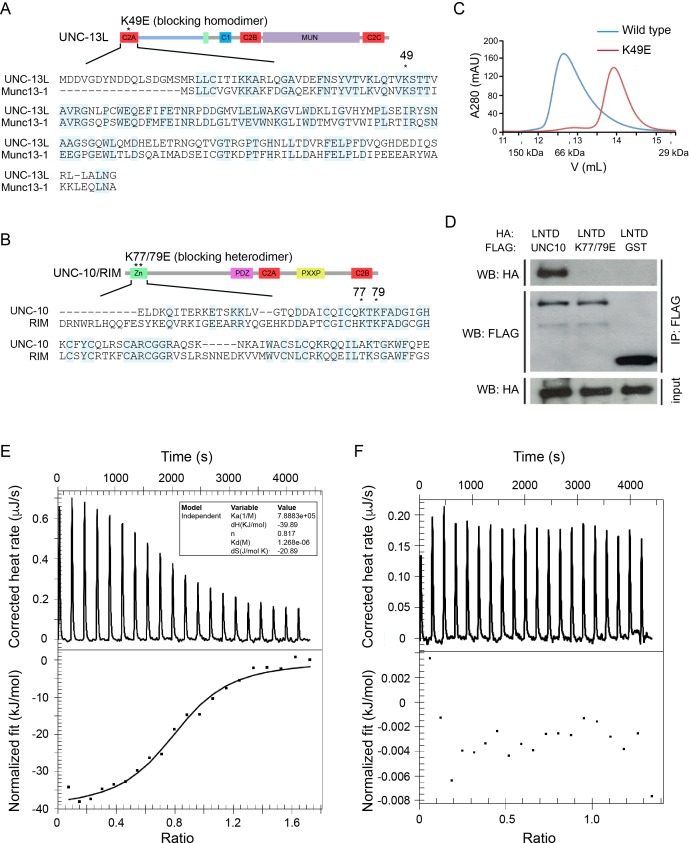
Figure 1. UNC-13L exhibits conserved homodimerization and heterodimerization in C. elegans.
(A, B) Sequence alignment of the C2A domain between worm UNC-13L and rat Munc13-1, and the zinc finger (ZF) domain between worm UNC-10 and mouse RIM1. The conserved residues in the C2A domain (K49) and the ZF domain (K77, K79) that disrupt homodimer and heterodimer formation are indicated by stars. (C) Separation of purified UNC-13L C2A monomer and homodimer by gel filtration. The wild-type C2A forms a homodimer that runs at 12.57 mL (blue trace, corresponding to an apparent weight of 89 kDa); this homodimer is disrupted in the monomeric UNC-13L(K49E) mutant which runs at 13.88 mL (apparent weight 52 kDa). Elution volumes of gel filtration standards are indicated underneath the plot, as follows: 11.62 mL for alcohol dehydrogenase (150 kDa), 12.85 mL for bovine serum albumin (66 kDa), and 15.45 mL for carbonic anhydrase (29 kDa), with the column void volume being 10.78 mL. (D) Co-immunoprecipitation (co-IP) shows that HA-LNTD (UNC-13L N-terminal domain, aa 1“605) binds wild-type FLAG-UNC-10 (aa 1–601) but not UNC-10(K77/79E) or GST. These results are representative of three independent experiments. (E, F) Isothermal titration calorimetry (ITC) analysis of binding of wild-type or mutated (K77/79E) UNC-10 with UNC-13L.