Abstract
Background
Parenteral nutrition solutions, artificial formulas, and human breast milk contain insufficient iodine to meet recommended intakes for preterm infants. Iodine deficiency may exacerbate transient hypothyroxinaemia in preterm infants and this may be associated with adverse neonatal and longer‐term outcomes.
Objectives
To assess the evidence from randomised controlled trials that dietary supplementation with iodine reduces mortality and morbidity in preterm infants.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 1), Ovid MEDLINE, Ovid Embase, Ovid Maternity & Infant Care Database, and CINAHL to February 2018. We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised or quasi‐randomised controlled trials that compared supplementing enteral or parenteral feeds with iodine (as iodide salt) versus placebo or no supplementation in preterm infants.
Data collection and analysis
Two review authors independently assessed trial eligibility and risk of bias, and extracted data. We analysed treatment effects as described in the individual trials and reported risk ratios (RR) and risk differences for dichotomous data, and mean differences (MD) for continuous data, with 95% confidence intervals (CI). We used a fixed‐effect model in meta‐analyses and planned to explore potential causes of heterogeneity in sensitivity analyses. We used the GRADE approach to assess the quality of evidence.
Main results
Two randomised controlled trials fulfilled the eligibility criteria. Both trials used methods to limit bias including allocation concealment and blinding of clinicians and investigators to the allocated intervention. The trials enrolled 1394 infants. One trial recruited 1273 participants. Most participants were born very preterm (less than 32 weeks' gestation) and about one‐third were extremely preterm (less than 28 weeks' gestation). Analyses found no effect of iodine supplementation on mortality before hospital discharge (typical RR 1.01, 95% CI 0.72 to 1.42; typical RD 0.00, 95% CI ‐0.03 to 0.03; 2 studies, 1380 infants) or on neurodevelopmental assessments at two years post‐term (Bayley Scales of Infant and Toddler Development, Third Edition main domain composite scores: cognitive: MD –0.30, 95% CI –2.44 to 1.84; motor: MD 0.20, 95% CI –2.15 to 2.55; language: MD –0.10, 95% CI –2.50 to 2.30; 1 study, 1259 infants). There were no differences in the proportion of infants who died or had a composite score less than 85 in any main Bayley domain (RR 1.05, 95% CI 0.94 to 1.17; RD 0.02 95% CI ‐0.03 to 0.08; 1 study, 1259 infants), or had visual impairment (RR 0.63, 95% CI 0.28 to 1.45; RD ‐0.01 95% CI ‐0.03 to 0.01; 1 study, 1092 infants) or auditory impairment (RR 1.05, 95% CI 0.51 to 2.16; RD 0.00 95% CI ‐0.02 to 0.02 1 study, 1093 infants). Using GRADE methods, we assessed the evidence for the effects on mortality and neurodevelopment outcomes as high‐certainty.
Authors' conclusions
The available trial data, predominantly from one large, high‐quality multicentre study published in 2017, do not show any evidence of beneficial effects of iodine supplementation for preterm infants. Given the high certainty of these estimates of effect, further trials of this intervention in this population are unlikely to be considered research priorities.
Plain language summary
Iodine supplementation for the prevention of mortality and adverse neurodevelopmental outcomes in preterm infants
Review question: Does giving preterm infants iodine supplements reduce their risk of dying or improve their brain development?
Background: Infants born preterm (several weeks early) may not receive the recommended amounts of iodine in their diet, as both preterm human breast milk and nutrition given via a drip in hospitals do not contain enough iodine to meet their heightened needs. Deficiency of iodine may affect the production of thyroid hormones that are important for brain and lung development in newborn infants. Given concerns that iodine deficiency may be harmful, we reviewed all the available evidence from clinical trials that assessed the effects of giving preterm infants iodine supplements.
Study characteristics: The evidence is up to date as of February 2018. We found two relevant trials (including 1394 infants). Both trials used reliable methods to ensure that their findings were not biased.
Key results: Analysis of data from these trials showed that iodine supplements for preterm infants does not affect the chances of dying or improve longer‐term brain development. This evidence was highly reliable.
Conclusions: The currently available evidence indicates that routine supplemental iodine does not have important benefits for preterm infants.
Summary of findings
Summary of findings for the main comparison. Iodine supplementation compared to control for the prevention of mortality and adverse neurodevelopmental outcomes in preterm infants.
| Iodine supplementation compared to control for the prevention of mortality and adverse neurodevelopmental outcomes in preterm infants | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with control | Risk difference with Iodine supplementation | ||||
| Mortality before hospital discharge | 1380 (2 RCTs) | ⊕⊕⊕⊕ High | RR 1.01 (0.72 to 1.42) | Study population | |
| 87 per 1000 | 1 more per 1000 (24 fewer to 37 more) | ||||
| Bayley‐III Cognitive composite score at 2 years of age corrected for prematurity | 1259 (1 RCT) | ⊕⊕⊕⊕ High | — | The mean Bayley‐III Cognitive composite score at 2 years of age corrected for prematurity was 89.2 | MD 0.3 lower (2.44 lower to 1.84 higher) |
| Bayley‐III Motor composite score at 2 years of age corrected for prematurity | 1259 (1 RCT) | ⊕⊕⊕⊕ High | — | The mean Bayley‐III Motor composite score at 2 years of age corrected for prematurity was 88 | MD 0.2 higher (2.15 lower to 2.55 higher) |
| Bayley‐III Language composite score at 2 years of age corrected for prematurity | 1259 (1 RCT) | ⊕⊕⊕⊕ High | — | The mean Bayley‐III Language composite score at 2 years of age corrected for prematurity was 85.2 | MD 0.1 lower (2.50 lower to 2.30 higher) |
| Low score in any main Bayley domain (< 85) or death | 1259 (1 RCT) | ⊕⊕⊕⊕ High | RR 1.05 (0.94 to 1.17) | Study population | |
| 490 per 1000 | 25 more per 1000 (29 fewer to 83 more) | ||||
|
Bayley‐III: Bayley Scales of Infant and Toddler Development, Third Edition; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Background
Description of the condition
Transient hypothyroxinaemia of prematurity is the more common thyroid dysfunction in preterm infants (Rooman 1996; Williams 2008; Delahunty 2010). It is characterised by a temporary postnatal reduction in plasma levels of free thyroxine (T4) and tri‐iodothyronine (T3). Unlike congenital hypothyroidism, plasma levels of thyroid‐stimulating hormone (TSH) remain normal or low (Williams 2008). The incidence, and degree and duration, of hypothyroxinaemia is inversely related to birth weight and gestational age and positively correlated with illness severity (particularly the severity of respiratory distress syndrome) (Reuss 1997). Transient hypothyroxinaemia, when defined as plasma T4 levels less than 40 µg/L, occurs in about 40% in infants born at 23 weeks' gestation and about 10% in infants born at 28 weeks' gestation (Reuss 1996).
Thyroid hormones play critical roles in postnatal transition processes and in foetal and infant growth and development (Forhead 2014; Velasco 2018). Thyroid hormones may act synergistically with corticosteroids to accelerate surfactant production and reduce the severity of respiratory distress syndrome and associated complications (need for mechanical ventilation, air leak, chronic lung disease (CLD)) in preterm infants (Liggins 1988). Thyroid hormones are essential for foetal and infant brain growth and development and concern exists that hypothyroxinaemia may impair neurodevelopment in preterm infants, and particularly in very or extremely preterm infants. Observational studies have suggested a link between transient hypothyroxinaemia and poorer neurodevelopmental outcomes including cognitive delay and cerebral palsy (Meijer 1992; Den Ouden 1996; Lucas 1996; Reuss 1996). However, some studies found no strong associations when potential confounders such as gestational age and illness severity are accounted for (Scratch 2014; Hollanders 2015; Hollanders 2016).
Iodine requirements
The aetiology of transient hypothyroxinaemia in preterm infants is multifactorial. As well as the contribution of non‐thyroidal illness and immaturity of the hypothalamic‐pituitary‐thyroid axis, iodine deficiency may contribute (Ares 1997; Murphy 2004). The neonatal thyroid gland is highly sensitive to iodine deficiency. Preterm infants do not have a substantial iodine pool and must renew its existing stores to meet thyroidal hormone requirements. Nutrient balance studies in healthy preterm infants indicate that daily iodine intakes of at least 30 µg/kg bodyweight are needed to maintain a positive balance (Ares 1997). One international consensus statement recommends daily iodine enteral intakes of 11 µg/kg to 55 µg/kg for healthy preterm infants (Agostoni 2010).
The typical iodine content of artificial formula ranges from about 70 to 250 μg/L (Belfort 2012). Maternal breast milk contains about 100 to 150 μg/L (depending on the dietary iodine intake of the mother), and commercially available breast‐milk fortifiers do not contain iodine (Koo 2017). Enterally fed preterm infants, therefore, may not achieve the recommended intake of iodine, especially during the first weeks of postnatal life when feed volumes are still being advanced (Ares 1997; Belfort 2012).
Preterm infants who are predominantly parenterally fed are at greater risk of negative iodine balance. Most commercially available parenteral nutrition solutions contain less iodine than breast milk or formula (Belfort 2012). The American Society for Clinical Nutrition has recommended daily parenteral iodine intakes of about 1 µg/kg (Belfort 2012). This conservative recommendation is influenced by an assumption that most parenterally fed preterm infants will absorb iodine transcutaneously from topical iodinated antiseptic solutions. However, the use of iodinated antiseptics for preterm infants has decreased due to concern that excessive transcutaneous iodine intake (greater than 100 µg/kg/day) may cause early acquired neonatal hypothyroidism (Smerdely 1989; Aitken 2014; Kieran 2018). Evidence exists that parentally fed very preterm infants who have not been exposed to other sources of iodine achieve daily iodine intakes of about 1 µg/kg to 3 µg/kg and are in negative iodine balance during the first week of postnatal life (Ibrahim 2003).
Description of the intervention
Iodine can be administered enterally as iodide salt and is well‐absorbed in the stomach and duodenum as well as being available for parenteral administration. Iodide is carried within the circulation, trapped in the thyroid follicle, and oxidised to iodine, which is then used to iodinate the tyrosine residues within thyroglobulin. Proteases cleave the residues to release thyroid hormones, which are formed through coupling of the iodotyrosyl residues (Forhead 2014).
How the intervention might work
Iodine deficiency may contribute to the pathogenesis of transient hypothyroxinaemia which may have adverse consequences for physiological postnatal transition and neurodevelopment during a critical phase of brain growth. Supplementing the diet or intake of preterm infants with iodide to meet recommended requirements could reduce the incidence, duration, and severity of transient hypothyrinoxinaemia and improve neurodevelopment and other outcomes.
Why it is important to do this review
Given the potential for negative iodine balance to cause hypothyroxinaemia and adverse neurodevelopmental and other outcomes, especially in very or extremely preterm infants, we reviewed the available evidence from randomised trials of iodine supplementation for preventing morbidity and mortality in preterm infants.
Objectives
To assess the evidence from randomised controlled trials that dietary supplementation with iodine reduces mortality and morbidity in preterm infants.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials using random or quasi‐random patient allocation. Cluster‐randomised trials (where the neonatal unit, rather than the individual patient, is randomised) were eligible for inclusion provided they met the other inclusion criteria.
Types of participants
Preterm infants (less than 37 weeks' gestation at birth).
Types of interventions
Supplementation with iodine (iodide: 30 µg/kg bodyweight per day or greater) compared with placebo or no supplementation. The comparison groups should have received the same nutrient input and other treatments apart from the level of iodide input.
Types of outcome measures
Primary outcomes
Neonatal mortality and mortality prior to hospital discharge.
Neurodevelopmental outcomes assessed by a validated test after 12 months' post‐term: neurological evaluations, developmental scores, and classifications of disability, including auditory and visual disability.
Secondary outcomes
-
Measures of the respiratory morbidity including:
duration of mechanical ventilation;
incidence of air leaks;
incidence of CLD (supplemental oxygen requirement at 36 weeks' postmenstrual age).
-
Other neonatal morbidity including incidence of:
necrotising enterocolitis (NEC);
retinopathy of prematurity (ROP);
persistent patent ductus arteriosus (PDA);
late‐onset infection.
Biochemical measures of thyroid function and iodine status such as plasma levels of T4 (free and total), T3, or TSH.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy).
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 1), Ovid MEDLINE (1946 to 27 February 2018), Ovid Embase (1974 to 27 February 2018), Ovid Maternity & Infant Care Database (1971 to February 2018), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 27 February 2018) using a combination of text words and MeSH terms described in Appendix 1. We limited the search outputs with the relevant search filters for clinical trials as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We applied no language restrictions.
We searched ClinicalTrials.gov and the World Health Organization's International Trials Registry and Platform (www.who.int/ictrp/en/) for completed or ongoing trials.
Searching other resources
We examined reference lists in previous reviews and included studies. We searched the proceedings of the annual meetings of the Pediatric Academic Societies (1993 to 2018), European Society for Paediatric Research (1995 to 2017), Royal College of Paediatrics and Child Health (2000 to 2018), and Perinatal Society of Australia and New Zealand (2000 to 2018). Trials reported only as abstracts were eligible if sufficient information was available from the report, or from contact with the authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
We screened the title and abstract of all studies identified by the search. Two review authors (VW, JVEB) independently assessed the full‐text articles of all potentially relevant trials. We excluded those studies that did not meet all of the inclusion criteria and we stated the reason for exclusion in the Characteristics of excluded studies table. We discussed any disagreements until consensus was achieved. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram.
Data extraction and management
Two review authors (VW, JVEB) independently extracted data using a data collection form to aid extraction of information on design, methodology, participants, interventions, outcomes, and treatment effects from each included study. We assessed the data collection form for usability prior to data extraction. We discussed any disagreements until we reached consensus. If data from the trial reports were insufficient, we contacted the trialists for further information.
Assessment of risk of bias in included studies
Two review authors (VW, JVEB) independently assessed the risk of bias (low, high, or unclear) of included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2017; Appendix 2):
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias).
We discussed any disagreements until we reached consensus or we consulted a third review author. See Appendix 2 for detailed description of risk of bias for each domain.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with 95% confidence intervals (CI). When it was deemed appropriate to combine two or more study arms, we obtained the treatment effects from the combined data using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We planned to determine the number needed to treat for an additional beneficial outcome (NNTB) or additional harmful outcome (NNTH) for a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials and the neonatal unit (or subunit) for cluster‐randomised trials. For cluster‐randomised trials, we planned to undertake analyses at the level of the participant while accounting for the clustering in the data using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Dealing with missing data
Where data were missing, and could not be derived, we attempted to:
contact the original study investigators to request the missing data;
where possible, impute missing standard deviations (SDs) using the coefficient of variation or calculate from other available statistics including standard errors, CIs, t values, and P values;
(if the data were thought to be missing at random) analyse the data without imputing any missing values;
(if this could not be assumed) impute the missing outcomes with replacement values, assuming all to have a poor outcome.
We planned sensitivity analyses to assess any changes in the direction or magnitude of effect resulting from data imputation.
Assessment of heterogeneity
Two review authors (VW, WM) assessed clinical heterogeneity with a meta‐analysis conducted only when both review authors agreed that study participants, interventions, and outcomes were sufficiently similar.
We examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies and described the percentage of variability in effect estimates that may have been due to heterogeneity rather than to sampling error. If we detected moderate or high heterogeneity (I² greater than 50%), we planned to explore the possible causes (e.g. differences in study design, participants, interventions, or completeness of outcome assessments).
Assessment of reporting biases
If more than 10 trials were included in a meta‐analysis, we planned to examine a funnel plot for asymmetry.
Data synthesis
We used a fixed‐effect model for meta‐analysis (as per Cochrane Neonatal recommendations). Where moderate or high heterogeneity existed, we planned to examine the potential causes in subgroup and sensitivity analyses.
Certainty of evidence
We assessed the certainty of evidence for the main comparisons at the outcomes level using the GRADE approach for the following outcomes: mortality and neurodevelopmental outcomes (Schünemann 2013; see Appendix 3).
Two review authors independently (VW, WM) assessed the certainty of the evidence for each of these outcomes. We considered evidence from randomised controlled trials as high quality but downgraded one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT to create a 'Summary of findings' table to report the certainty of the evidence.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses by:
gestation: extremely preterm (less than 28 weeks' gestation) versus very preterm (28 to 31 weeks' gestation) infants versus preterm infants (32 weeks' gestation or later);
indication: prophylactic versus therapeutic use (to treat iodine deficiency or hypothyroxinaemia).
Sensitivity analysis
We planned sensitivity analyses to determine if the findings were affected by including only studies of adequate methodology (low risk of bias), defined as adequate randomisation and allocation concealment, blinding of intervention and measurement, and less than 10% loss to follow‐up.
Results
Description of studies
Results of the search
See Figure 1.
1.
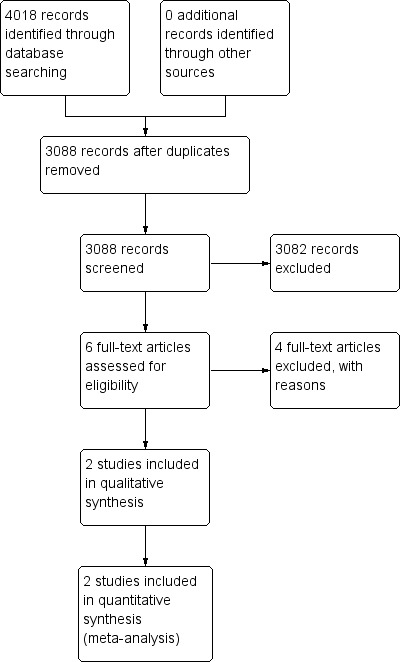
Study flow diagram.
We included one new trial (Williams 2017), in addition to the existing trial (Rogahn 2000).
We identified no ongoing trials.
Included studies
Rogahn 2000: the investigators randomly allocated 121 infants born at less than 33 weeks' gestation to receive either iodide‐supplemented (272 μg/L) formula or the same formula without iodide supplementation. This strategy was designed to provide an iodine intake of 40 μg/kg/day to 50 μg/kg/day in the intervention group versus 12 μg/kg/day to 16 μg/kg/day in the control group. Infants were allocated to receive the trial formulas until 40 weeks' postmenstrual age. The primary outcomes (on which the sample size calculation was based) were the plasma levels of thyroid hormones. Some inhospital clinical outcome data were reported. Neurodevelopment and cognitive function were not assessed.
Williams 2017: the investigators randomly allocated 1273 infants born at less than 31 weeks' gestation to receive either sodium iodide (75 μg/L) or sodium chloride (75 μg/L) given parenterally or enterally (as indicated) at a dose of 30 μg/kg/day. Infants were recruited less than 42 hours after birth and were allocated to receive the trial solution daily until 34 weeks' postmenstrual age. The primary outcome was neurodevelopmental status defined by the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley‐III) main domains (cognitive, motor, language) at two years' post‐term. A minimum Bayley‐III score for each domain was awarded if an infant died before neurodevelopmental assessment or was too disabled to make an assessment meaningful. Secondary outcomes were the subtests of the Bayley‐III; Bayley‐III analysed as a dichotomous outcome (death or a Bayley‐III score less than 85 in any of the main domains versus a Bayley‐III score 85 or greater); plasma levels of T4, TSH, and thyroid‐binding globulin (TBG) on postnatal days 7, 14, 28, and at 34 weeks' gestation; incidence of NEC, ROP, PDA, CLD, and late‐onset infection; and prescribed drug usage.
Excluded studies
We excluded five studies (Bouhouch 2013; Wang 2013; Belykh 2014; Kartoğlu 2014; Mohammed 2015). Reasons for exclusion are presented in the Characteristics of excluded studies table.
Risk of bias in included studies
2.
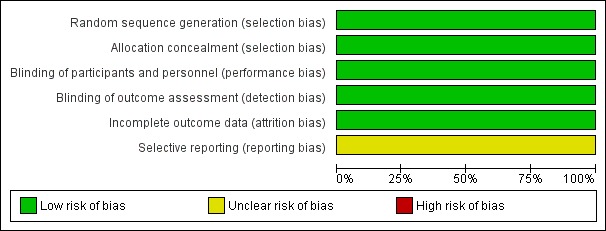
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Methodological quality was generally high.
Allocation
Rogahn 2000: the random sequence was derived from a random number table and concealed within sealed opaque envelopes (low risk).
Williams 2017: randomisation was achieved through a secure website and used a bespoke minimisation algorithm to ensure balance across cross groups for recruiting site, gender, and gestational age. Infants from multiple births were assigned individually (low risk).
Blinding
Rogahn 2000: the intervention and control formula milks were identical in appearance and the iodine levels were not known to parents, caregivers, or outcome assessors (low risk).
Williams 2017: the intervention and control solutions were identical in appearance and the research team members, parents, neonatal unit staff, and pharmacy staff were blinded (low risk).
Incomplete outcome data
Rogahn 2000: complete outcome data up to 40 weeks' postmenstrual age were available for 114/121 infants. Biochemical data were not available for six infants withdrawn from the study (reasons not stated) and for one infant who died during the study period. The primary analyses were by intention‐to‐treat (low risk).
Williams 2017: outcome assessment was achieved for 631/637 infants in the iodide supplementation group and 628/636 infants in the control group. Fourteen infants (six infants in the iodide supplementation group and eight infants in the placebo group) were withdrawn with no data available for analyses (low risk).
Selective reporting
Rogahn 2000: the published study did not report number of days of continuous positive airways pressure, number of days until completing the study, duration of parenteral feeding and human milk feeding in days, and daily milk intake (unclear risk).
Williams 2017: secondary outcomes were reported as per the protocol with the exception of days of endotracheal intubation and days of continuous positive airways pressure (unclear risk).
Effects of interventions
See: Table 1
1. Mortality (outcome 1.1)
Meta‐analysis showed no significant difference in the incidence of death before hospital discharge: typical RR 1.01 (95% CI 0.72 to 1.42); typical RD 0.00 (95% CI –0.03 to 0.03); I² = 0%, 2 trials, 1380 participants; high‐certainty evidence (Analysis 1.1; Figure 4).
1.1. Analysis.
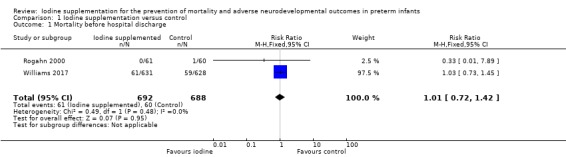
Comparison 1 Iodine supplementation versus control, Outcome 1 Mortality before hospital discharge.
4.
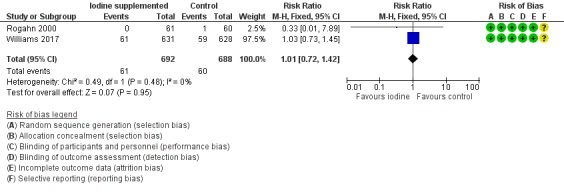
Forest plot of comparison: 1 Iodine supplementation versus control, outcome: 1.1 Mortality before hospital discharge.
2. Neurodevelopmental outcomes (outcomes 1.2 to 1.7)
Rogahn 2000 did not assess neurodevelopmental outcomes.
Williams 2017 found no significant difference in Bayley‐III composite scores across the three domains:
Cognitive: MD –0.30 (95% CI –2.44 to 1.84); 1 trial, 1259 participants; high‐certainty evidence (Analysis 1.2; Figure 5);
Motor: MD 0.20 (95% CI –2.15 to 2.55); 1 trial, 1259 participants; high‐certainty evidence (Analysis 1.3; Figure 6);
Language: MD –0.10 (95% CI –2.50 to 2.30); 1 trial, 1259 participants; high‐certainty evidence (Analysis 1.4; Figure 7)
1.2. Analysis.
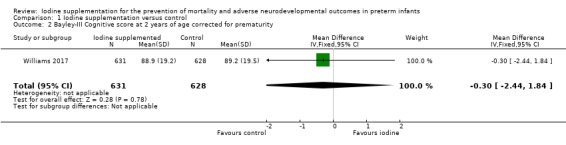
Comparison 1 Iodine supplementation versus control, Outcome 2 Bayley‐III Cognitive score at 2 years of age corrected for prematurity.
5.
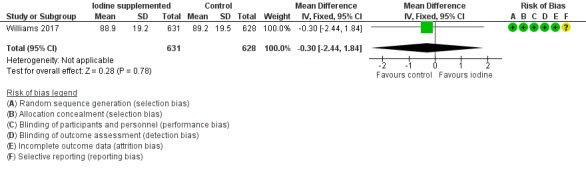
Forest plot of comparison: 1 Iodine supplementation versus control, outcome: 1.2 Bayley‐III Cognitive score at 2 years of age corrected for prematurity.
1.3. Analysis.
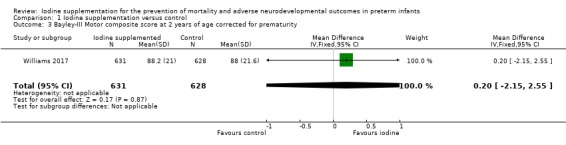
Comparison 1 Iodine supplementation versus control, Outcome 3 Bayley‐III Motor composite score at 2 years of age corrected for prematurity.
6.
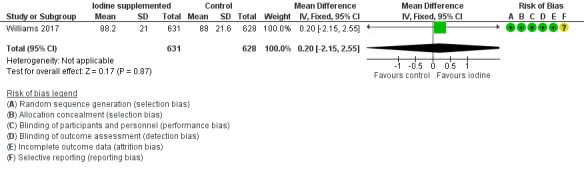
Forest plot of comparison: 1 Iodine supplementation versus control, outcome: 1.3 Bayley‐III Motor composite score at 2 years of age corrected for prematurity.
1.4. Analysis.
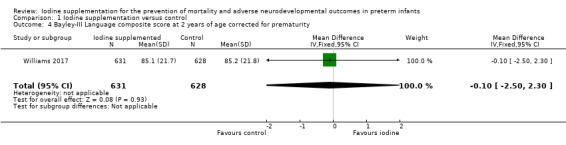
Comparison 1 Iodine supplementation versus control, Outcome 4 Bayley‐III Language composite score at 2 years of age corrected for prematurity.
7.
Forest plot of comparison: 1 Iodine supplementation versus control, outcome: 1.4 Bayley‐III Language composite score at 2 years of age corrected for prematurity.
There were no differences in the proportion of infants who died or had a composite score less than 85 in any main Bayley domain (RR 1.05, 95% CI 0.94 to 1.17; RD 0.02 95% CI ‐0.03 to 0.08; 1 study, 1259 participants; high‐certainty evidence)(Analysis 1.5; Figure 8).
1.5. Analysis.
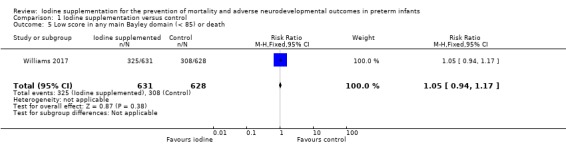
Comparison 1 Iodine supplementation versus control, Outcome 5 Low score in any main Bayley domain (< 85) or death.
8.
Forest plot of comparison: 1 Iodine supplementation versus control, outcome: 1.5 Low score in any main Bayley domain (less than 85) or death.
Williams 2017 found no differences in rates of:
visual impairment: RR 0.63 (95% CI 0.28 to 1.45); RD ‐0.01 (95% CI ‐0.03 to 0.01); 1 study, 1092 participants (Analysis 1.6);
auditory impairment: RR 1.05 (95% CI 0.51 to 2.16); RD 0.00 (95% CI ‐0.02 to 0.02); 1 study, 1093 participants. (Analysis 1.7).
1.6. Analysis.
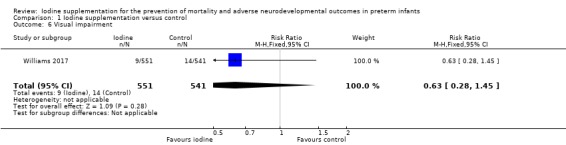
Comparison 1 Iodine supplementation versus control, Outcome 6 Visual impairment.
1.7. Analysis.
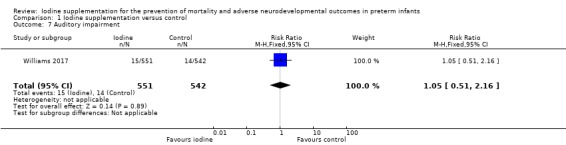
Comparison 1 Iodine supplementation versus control, Outcome 7 Auditory impairment.
3. Respiratory morbidity (outcomes 1.8 to 1.9)
Rogahn 2000 found no difference in the duration of mechanical ventilation: median difference 0 days (SD not reported). Williams 2017 did not report the duration of mechanical ventilation.
Williams 2017 found a lower incidence of air leaks (pneumothorax) in the iodide group: RR 0.50 (95% CI 0.31 to 0.80); RD –0.04 (95% CI –0.07 to –0.01); NNTH 25 (95% CI 14 to 100); 1 trial, 1257 participants (Analysis 1.8). Rogahn 2000 did not report the incidence of air leaks.
1.8. Analysis.
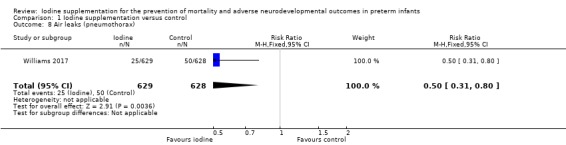
Comparison 1 Iodine supplementation versus control, Outcome 8 Air leaks (pneumothorax).
Meta‐analyses found no significant differences in:
CLD: typical RR 1.07 (95% CI 0.94 to 1.22); typical RR 0.03 (95% CI ‐0.03 to 0.08); I² = 75%, 2 trials, 1377 participants (Analysis 1.9).
1.9. Analysis.
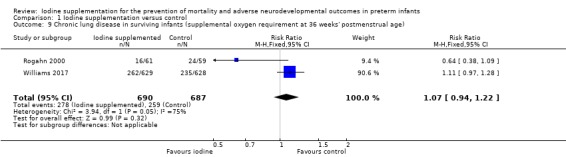
Comparison 1 Iodine supplementation versus control, Outcome 9 Chronic lung disease in surviving infants (supplemental oxygen requirement at 36 weeks' postmenstrual age).
4. Other neonatal morbidity (outcomes 1.10 to 1.13)
Williams 2017 found no differences in the incidences of:
NEC: RR 1.24 (95% CI 0.98 to 1.56); RD 0.04 (95% CI ‐0.00 to 0.08); 1 trial, 1259 participants (Analysis 1.10);
ROP: RR 0.97 (95% CI 0.64 to 1.49); RD ‐0.00 (95% CI ‐0.03 to 0.03); 1 trial,1257 participants (Analysis 1.11);
PDA: RR 0.98 (95% CI 0.83 to 1.16); RD ‐0.01 (95% CI ‐0.06 to 0.04); 1 trial,1259 participants (Analysis 1.12);
Late‐onset infection: RR 1.09 (95% CI 0.96 to 1.24); RD 0.04 (95% CI ‐0.02 to 0.09); 1 trial,1259 participants (Analysis 1.13).
1.10. Analysis.
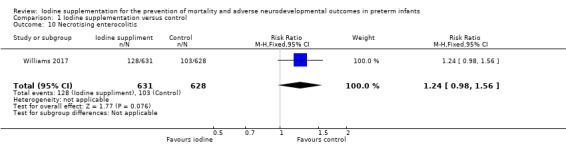
Comparison 1 Iodine supplementation versus control, Outcome 10 Necrotising enterocolitis.
1.11. Analysis.
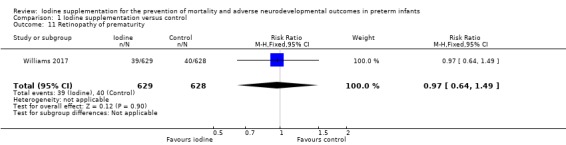
Comparison 1 Iodine supplementation versus control, Outcome 11 Retinopathy of prematurity.
1.12. Analysis.
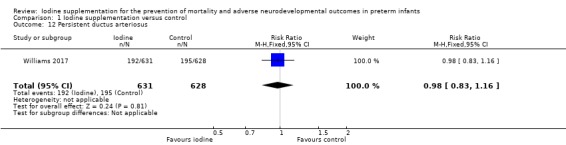
Comparison 1 Iodine supplementation versus control, Outcome 12 Persistent ductus arteriosus.
1.13. Analysis.
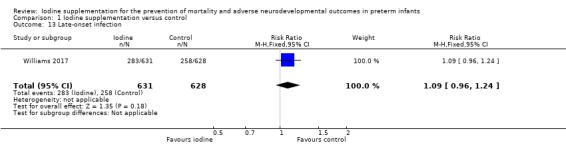
Comparison 1 Iodine supplementation versus control, Outcome 13 Late‐onset infection.
Rogahn 2000 did not report the incidence of NEC, PDA, ROP, or late‐onset infection.
5. Biochemical measures of thyroid function (outcomes 1.14 to 1.15)
Rogahn 2000 did not report any statistically significant differences in the plasma levels of T4, T3, or TSH measured up to 40 weeks' postmenstrual age.
Williams 2017 did not report any significant differences in plasma levels of T4, TSH, or TBG up to 34 to 36 weeks' postmenstrual age.
Meta‐analyses found no significant differences at 34 to 36 weeks' postmenstrual age in plasma levels of:
total T4: MD 0.29 (95% CI –1.51 to 2.09) nmol/L; I² = 0%, 2 trials, 1121 participants (Analysis 1.14);
TSH: MD 0.05 (95% CI –0.09 to 0.18) mU/L; I² = 8%, 2 trials, 1119 participants (Analysis 1.15).
1.14. Analysis.
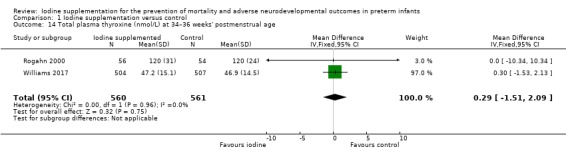
Comparison 1 Iodine supplementation versus control, Outcome 14 Total plasma thyroxine (nmol/L) at 34–36 weeks' postmenstrual age.
1.15. Analysis.
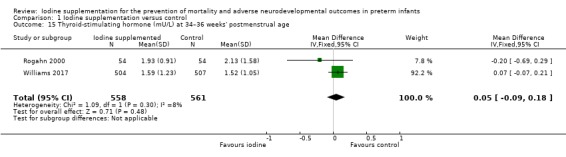
Comparison 1 Iodine supplementation versus control, Outcome 15 Thyroid‐stimulating hormone (mU/L) at 34–36 weeks' postmenstrual age.
Subgroup analyses
Both trials assessed the effect of prophylactic rather than therapeutic iodine supplementation. Williams 2017 reported that a prespecified subgroup analysis of infants who had hypothyroxinaemia (plasma T4 less than 10th percentile on postnatal day 7, 14, or 28; 288 participants) versus infants without hypothyroxinaemia found no significant differences in scores across the Bayley‐III main domains.
All participants in Williams 2017 were born before 31 weeks' gestation and those in Rogahn 2000 before 33 weeks' gestation. The data available did not allow further subgroup analyses by birth weight and gestational age to be undertaken. Williams 2017 reported that there was no evidence of a difference in the effect of iodide versus placebo on the mean Bayley‐III main domain scores by gestational age groups (less than 26, 26 to 27, or 28 to 30 weeks).
Discussion
Summary of main results
We identified two eligible randomised controlled trials that included 1394 preterm infants born before 33 weeks' gestation. Most infants (1273/1394) participated in one large multicentre trial undertaken in the UK during the past decade (Williams 2017). These trials found no evidence that iodine supplementation affected the incidence of mortality, adverse neurodevelopmental outcomes, or major neonatal morbidity in preterm infants.
Overall completeness and applicability of evidence
The previous version of this review included one small randomised controlled trial (121 participants; Rogahn 2000). The available data were insufficient to determine whether iodine supplementation affected mortality and morbidity in preterm infants. The review concluded that data from "a large pragmatic randomised controlled trial [were needed] to determine if iodine supplementation, started within a few days after birth, prevents morbidity and mortality in preterm infants" (Ibrahim 2006). One large multicentre randomised controlled trial has now provided these data (Williams 2017). Inclusion of this trial in the review increased the total number of participants included in assessments of iodine supplementation from 121 to 1394. This included a substantial proportion (34%) of extremely preterm (less than 28 weeks' gestation) infants, the group at greatest risk of transient hypothyroxinaemia.
Data on neurodevelopmental outcomes, which were not reported by Rogahn 2000, were available from Williams 2017. These assessments used a validated and accepted tool (Bayley‐III). Assessors were unaware of the groups to which the participants had been allocated. Outcome data were available for more than 90% of the cohort (balanced across the intervention and placebo groups). The analyses found no effects of iodine supplementation on the composite scores for Bayley‐III assessments in major domains. The 95% CI for the estimates of effect size estimates were narrow indicating that modest, though potentially important, effects on motor, language, or cognitive development was unlikely to have been missed. We were unable to undertake a subgroup analysis of extremely preterm or extremely low birth weight infants with the available data. However, Williams 2017 reported that the lack of effect of iodine supplementation on mortality and neurodevelopment was consistent across gestational age bands (less than 26, 26 to 27, or 28 to 30 weeks).
Meta‐analyses of the effect on major neonatal morbidity (NEC, CLD, ROP, late‐onset infection) found no evidence of benefit of supplemental iodine versus placebo. Data from Williams 2017 indicated a substantial reduction in the risk of pneumothorax associated with iodine supplementation. However, given this was one of many secondary outcomes analysed, this finding should be interpreted with caution.
Both of the included trials assessed the effect of prophylactic (empirical) iodine supplementation rather than therapeutic (indicated) supplementation for infants with a diagnosis of hypothyroxinaemia, the group for whom the putative benefits of the intervention might be most likely (Williams 2011; van Wassenaer‐Leemhuis 2014). Williams 2017 reported a prespecified subgroup analysis that found no difference in the effect size in those infants with versus without evidence of hypothyroxinaemia. This finding is consistent with data from both trials which showed that iodine supplementation had no effect of biochemical measures of thyroid function including plasma levels of T4 or TSH.
Quality of the evidence
The included trials were high quality with both using methods to limit bias including ensuring allocation concealment and blinding of parents, clinicians, and investigators. Adherence to the intervention was high. In Williams 2017, less than 5% of infants were withdrawn from the trial during the intervention period (and their data were collected for reporting of intention‐to‐treat analyses). Both trials achieved near‐complete primary outcome assessment. The meta‐analyses (where data from both trials could be combined) were dominated by the larger trial but none of the analyses had evidence of statistical heterogeneity. Analyses generated precise and consistent effect size estimates that indicate that important effects were unlikely to have been missed. Consequently, the overall certainty of the evidence as assessed by GRADE methods was high for the primary outcomes (Table 1).
Potential biases in the review process
The main concern with the review process was that the findings may have been subjected to publication or other reporting biases. To minimise potential publication bias, we included non‐English language publications and we searched conference proceedings and trial registries for unreported, unpublished, or ongoing trials. As only two trials were eligible in our review, however, there were insufficient data to explore potential publication or reporting bias through examination of asymmetry in funnel plots.
Agreements and disagreements with other studies or reviews
Cochrane Reviews of trials of postnatal thyroid hormone supplementation, either prophylactically or targeted at infants with transient hypothyroxinaemia, found no evidence of effect on mortality or neurodevelopment, or on the severity of respiratory distress syndrome or the incidence of CLD, in very preterm infants (Osborn 2007a; Osborn 2007b).
Authors' conclusions
Implications for practice.
The available trial data indicate that iodine supplementation does not reduce mortality and morbidity in very preterm infants.
Implications for research.
There is no longer a need for further randomised controlled trials to determine if iodine supplementation, started within a few days after birth, prevents morbidity and mortality in preterm infants.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2019 | Amended | Minor edits to wording in abstract and PLS. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 18 February 2019 | New search has been performed | Updated to include new trial and newer methods, including 'Summary of findings' table. |
| 7 June 2018 | New citation required and conclusions have changed | One new trial included (Williams 2017). |
| 18 September 2008 | Amended | Converted to new review format. |
| 14 February 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Fiona Williams for kindly providing further data for Williams 2017.
Appendices
Appendix 1. Search strategy
Databases searched
CENTRAL 459 records
MEDLINE 1887 records
Embase 1292 records
Maternity & Infant Care 27 records
CINAHL 353 records
[Trials registers: ClinicalTrials.gov and WHO ICTRP (45 and 14 records)]
MEDLINE
Via Ovid
Search date 27 February 2018
1887 records
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
1 Iodine/ (24063)
2 Iodine$.ti,ab. (43673)
3 Iodides/ (9638)
4 Iodide$.ti,ab. (31252)
5 Potassium Iodide/ (1961)
6 exp Thyroid Hormones/ (61903)
7 (thyroid adj2 hormone$).ti,ab. (41896)
8 exp dextrothyroxine/ or exp diiodotyrosine/ or exp monoiodotyrosine/ or exp "thyroid (usp)"/ or exp thyronines/ or exp thyroxine/ or triiodothyronine/ (47871)
9 dextrothyroxine$.ti,ab. (82)
10 diiodotyrosine$.ti,ab. (384)
11 monoiodotyrosine$.ti,ab. (233)
12 (thyroid adj2 usp).ti,ab. (10)
13 thyronine$.ti,ab. (1493)
14 thyroxine$.ti,ab. (25721)
15 L‐thyroxine therap$.ti,ab. (212)
16 hypothyroxin$.ti,ab. (535)
17 triiodothyronine$.ti,ab. (15979)
18 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (162174)
19 exp Infant, Newborn/ (561814)
20 Premature Birth/ (10565)
21 (neonat$ or neo nat$).ti,ab. (235139)
22 (newborn$ or new born$ or newly born$).ti,ab. (151502)
23 (preterm or preterms or pre term or pre terms).ti,ab. (62750)
24 (preemie$ or premie or premies).ti,ab. (145)
25 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (14033)
26 (low adj3 (birthweight$ or birth weight$)).ti,ab. (30911)
27 (lbw or vlbw or elbw).ti,ab. (7222)
28 infan$.ti,ab. (392750)
29 (baby or babies).ti,ab. (62792)
30 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 (961691)
31 18 and 30 (8059)
32 randomized controlled trial.pt. (454398)
33 controlled clinical trial.pt. (92180)
34 randomized.ab. (404232)
35 placebo.ab. (186767)
36 drug therapy.fs. (1995838)
37 randomly.ab. (285844)
38 trial.ab. (419845)
39 groups.ab. (1768325)
40 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 (4149324)
41 exp animals/ not humans.sh. (4429349)
42 40 not 41 (3585184)
43 31 and 42 (1892)
Embase
Via Ovid
Search date 27 February 2018
1292 records
Database: Embase <1974 to 2018 Week 09>
1 Iodine/ (40146)
2 Iodine$.ti,ab. (53091)
3 Iodide/ (10272)
4 Iodide$.ti,ab. (38417)
5 Potassium Iodide/ (4652)
6 thyroid hormone/ (30585)
7 (thyroid adj2 hormone$).ti,ab. (51589)
8 dextrothyroxine/ (525)
9 diiodotyrosine/ (863)
10 Iodotyrosine/ (805)
11 "thyroid usp"/ (590)
12 thyroxine/ (52309)
13 Iodothyronine/ (846)
14 thyroid extract/ (639)
15 thyronine derivative/ (399)
16 dextrothyroxine$.ti,ab. (79)
17 diiodotyrosine$.ti,ab. (387)
18 monoiodotyrosine$.ti,ab. (253)
19 (thyroid adj2 usp).ti,ab. (11)
20 thyronine$.ti,ab. (1544)
21 thyroxine$.ti,ab. (29804)
22 L‐thyroxine therap$.ti,ab. (282)
23 hypothyroxin$.ti,ab. (703)
24 triiodothyronine$.ti,ab. (17472)
25 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (208650)
26 Newborn/ (535958)
27 Prematurity/ (91912)
28 (neonat$ or neo nat$).ti,ab. (302978)
29 (newborn$ or new born$ or newly born$).ti,ab. (184831)
30 (preterm or preterms or pre term or pre terms).ti,ab. (86501)
31 (preemie$ or premie or premies).ti,ab. (214)
32 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (18987)
33 (low adj3 (birthweight$ or birth weight$)).ti,ab. (38449)
34 (lbw or vlbw or elbw).ti,ab. (9722)
35 infan$.ti,ab. (460657)
36 (baby or babies).ti,ab. (85582)
37 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 (1082447)
38 25 and 37 (11309)
39 (random* or factorial* or placebo* or assign* or allocat* or crossover*).tw. (1659186)
40 (cross adj over*).tw. (28819)
41 (trial* and (control* or comparative)).tw. (503252)
42 ((blind* or mask*) and (single or double or triple or treble)).tw. (234368)
43 (treatment adj arm*).tw. (16210)
44 (control* adj group*).tw. (550749)
45 (phase adj (III or three)).tw. (51245)
46 (versus or vs).tw. (1733178)
47 rct.tw. (27329)
48 Crossover Procedure/ (54400)
49 Double Blind Procedure/ (146612)
50 Single Blind Procedure/ (30461)
51 Randomization/ (77056)
52 Placebo/ (319921)
53 exp Clinical Trial/ (1295328)
54 Parallel Design/ (7954)
55 Latin Square Design/ (359)
56 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 (4258513)
57 exp animal/ or exp nonhuman/ or exp animal experiment/ or exp animal model/ (25585217)
58 exp human/ (19276078)
59 57 not 58 (6309139)
60 56 not 59 (3698276)
61 38 and 60 (1292)
CENTRAL
Via Wiley’s Cochrane Library
Search date 27 February 2018
Records retrieved= 459
#1 MeSH descriptor: [Iodine] explode all trees
#2 iodine*:ti,ab,kw (Word variations have been searched)
#3 MeSH descriptor: [Iodides] explode all trees
#4 iodide*:ti,ab,kw (Word variations have been searched)
#5 MeSH descriptor: [Potassium Iodide] explode all trees
#6 MeSH descriptor: [Thyroid Hormones] explode all trees
#7 thyroid near/2 hormone*:ti,ab,kw (Word variations have been searched)
#8 MeSH descriptor: [Dextrothyroxine] explode all trees
#9 MeSH descriptor: [Diiodotyrosine] explode all trees
#10 MeSH descriptor: [Monoiodotyrosine] explode all trees
#11 MeSH descriptor: [Thyroid (USP)] explode all trees
#12 MeSH descriptor: [Thyronines] explode all trees
#13 MeSH descriptor: [Thyroxine] explode all trees
#14 MeSH descriptor: [Triiodothyronine] explode all trees
#15 dextrothyroxine*:ti,ab,kw or diiodotyrosine*:ti,ab,kw or monoiodotyrosine*:ti,ab,kw or thyroid near/2 usp:ti,ab,kw or thyronine*:ti,ab,kw (Word variations have been searched)
#16 thyroxine*:ti,ab,kw or L‐thyroxine therap*:ti,ab,kw or Hypothyroxin*:ti,ab,kw or triiodothyronine*:ti,ab,kw (Word variations have been searched)
#17 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 MeSH descriptor: [Infant, Newborn] explode all trees
#19 MeSH descriptor: [Premature Birth] explode all trees
#20 neonat*:ti,ab,kw (Word variations have been searched)
#21 neo‐nat*:ti,ab,kw (Word variations have been searched)
#22 newborn or new born* or newly born*:ti,ab,kw (Word variations have been searched)
#23 preterm or preterms or (pre term) or (pre terms):ti,ab,kw (Word variations have been searched)
#24 preemie* or premie or premies:ti,ab,kw (Word variations have been searched)
#25 prematur* near/3 (birth* or born or deliver*):ti,ab,kw (Word variations have been searched)
#26 low near/3 (birthweight* or birth weight*):ti,ab,kw (Word variations have been searched)
#27 lbw or vlbw or elbw:ti,ab,kw (Word variations have been searched)
#28 infan* or baby or babies:ti,ab,kw (Word variations have been searched)
#29 #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28
#30 #17 and #29
Maternity & Infant Care
Via Ovid
Search date 27 February 2018
Records retrieved= 27
Database: Maternity & Infant Care Database (MIDIRS) <1971 to December 2017>
1 Iodine.de. (102)
2 iodine$.ti,ab. (402)
3 iodides.de. (4)
4 iodide$.ti,ab. (28)
5 potassium iodide$.de,ti,ab. (0)
6 thyroid hormone$.de,ti,ab. (323)
7 dextrothyroxine.de,ti,ab. (0)
8 diiodotyrosine.de,ti,ab. (0)
9 monoiodotyrosine.de,ti,ab. (0)
10 (thyroid adj2 usp).de,ti,ab. (0)
11 thyronines.de,ti,ab. (0)
12 thyroxine.de,ti,ab. (355)
13 triiodothyronine.de,ti,ab. (79)
14 L‐thyroxine therap$.de,ti,ab. (3)
15 hypothyroxin$.de,ti,ab. (92)
16 triiodothyronine$.de,ti,ab. (79)
17 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 (905)
18 limit 17 to randomised controlled trial (27)
CINAHL
Via EBSCO
Search date 27 February 2018
Records retrieved = 353 records
| S1 | (MH “iodine”) Results 1721 | |
| S2 | TX iodine* |
View Results (5,940) View Details Edit |
| S3 | (MH "Iodides+") OR (MH "Potassium Iodide") |
View Results (219) View Details Edit |
| S4 | TX iodide* |
View Results (958) View Details Edit |
| S5 | (MH "Thyroid Hormones+") |
View Results (3,047) View Details Edit |
| S6 | TX thyroid N2 hormone* |
View Results (3,248) View Details Edit |
| S7 | (MH "Thyronines+") |
View Results (412) View Details Edit |
| S8 | (MH "Thyroxine+") |
View Results (1,896) View Details Edit |
| S9 | (MH "Triiodothyronine") |
View Results (399) View Details Edit |
| S10 | TX dextrothyroxine* OR TX diiodotyrosine* OR TX monoiodotyrosine* OR TX thyroid N2 usp OR TX thyronine* OR TX thyroxine* OR TX L‐thyroxine N2 therap* OR TX hypothyroxin* OR TX triiodothyronine* |
View Results (2,611) View Details Edit |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 |
View Results (10,887) View Details Edit |
| S12 | (MH "Infant, Newborn+") |
Rerun View Details Edit |
| S13 | TX ( (neonat* or neo nat*) ) OR TX ( (newborn* or new born* or newly born*) ) OR TX ( (preterm or preterms or pre term or pre terms) ) OR TX ( (preemie$ or premie or premies) ) OR TX ( (prematur* NEAR/3 (birth* or born or deliver*)) ) OR TX ( (low NEAR/3 (birthweight* or birth weight*)) ) OR TX ( (lbw or vlbw or elbw) ) OR TX infan* OR TX ( (baby or babies) ) |
Rerun View Details Edit |
| S14 | S12 OR S13 |
Rerun View Details Edit |
| S15 | S11 AND S14 |
View Results (1,376) View Details Edit |
| S16 | (MH "Randomized Controlled Trials") OR (MH "Clinical Trials+") |
View Results (235,417) View Details Edit |
| S17 | TI randomiz* OR AB randomiz* OR TI randomis* OR AB randomis* OR AB placebo OR AB randomly OR AB trial OR AB groups |
View Results (664,541) View Details Edit |
| S18 | S16 OR S17 |
View Results (760,646) View Details Edit |
| S19 | S15 AND S18 |
View Results (353) View Details |
Trials registers ‐ ClinicalTrials.gov and WHO ICTRP
Clinicaltrials.gov
https://clinicaltrials.gov/ct2/home
Search date 27 February 2018
45 unique records identified
Two searches
Search one
Intervention search = Dietary supplement: Iodine, limited to child and interventional studies
Search two
Condition search = Iodine deficiency, limited to child and interventional studies
WHO ICTRP
http://apps.who.int/trialsearch/Default.aspx
Search date 27 February 2018
14 unique records
Search 1 Iodine deficiency [condition] 9 records for 8 trials
Search 2 ((pregnant OR pregnancy OR prenatal OR women OR woman OR mother OR mothers OR maternal OR lactation OR lactating [title]) AND (Iodine [intervention])) 7 records
Appendix 2. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we reincluded missing data in the analyses. We categorised the methods as:
low risk (less than 20% missing data);
high risk (20% or greater missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Appendix 3. GRADE
GRADE considers that evidence from randomised controlled trials is 'high' quality but that assessment may be downgraded based on consideration of any of five areas:
design (risk of bias);
consistency across studies;
directness of the evidence;
precision of estimates; and
presence of publication bias.
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Data and analyses
Comparison 1. Iodine supplementation versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality before hospital discharge | 2 | 1380 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.72, 1.42] |
| 2 Bayley‐III Cognitive score at 2 years of age corrected for prematurity | 1 | 1259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.44, 1.84] |
| 3 Bayley‐III Motor composite score at 2 years of age corrected for prematurity | 1 | 1259 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.15, 2.55] |
| 4 Bayley‐III Language composite score at 2 years of age corrected for prematurity | 1 | 1259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.50, 2.30] |
| 5 Low score in any main Bayley domain (< 85) or death | 1 | 1259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.94, 1.17] |
| 6 Visual impairment | 1 | 1092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.28, 1.45] |
| 7 Auditory impairment | 1 | 1093 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.51, 2.16] |
| 8 Air leaks (pneumothorax) | 1 | 1257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.31, 0.80] |
| 9 Chronic lung disease in surviving infants (supplemental oxygen requirement at 36 weeks' postmenstrual age) | 2 | 1377 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.94, 1.22] |
| 10 Necrotising enterocolitis | 1 | 1259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.98, 1.56] |
| 11 Retinopathy of prematurity | 1 | 1257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.64, 1.49] |
| 12 Persistent ductus arteriosus | 1 | 1259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.16] |
| 13 Late‐onset infection | 1 | 1259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] |
| 14 Total plasma thyroxine (nmol/L) at 34–36 weeks' postmenstrual age | 2 | 1121 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐1.51, 2.09] |
| 15 Thyroid‐stimulating hormone (mU/L) at 34–36 weeks' postmenstrual age | 2 | 1119 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.09, 0.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Rogahn 2000.
| Methods | Randomised controlled trial Study sites: 5 neonatal units in the Mersey region, UK (1997–1998) |
|
| Participants | Newborn infants < 33 weeks' gestation at birth | |
| Interventions | Treatment (n = 61): iodide supplemented "preterm" formula milk (272 μg/L) Control (n = 60): same milk without iodine supplementation: iodine concentration 68 μg/L Allocated formula continued until infants reached 40 to 41 weeks' postmenstrual age. |
|
| Outcomes | Primary: plasma levels of TSH, total thyroxine, total tri‐iodothyronine, and, when possible, T4 Secondary: volume of milk ingested; weekly change in weight, leg length, and head circumference during trial; mortality; respiratory outcomes (days ventilated, days requiring supplemental oxygen therapy, incidence of CLD); incidence of severe intraventricular haemorrhage and cystic periventricular leukomalacia; duration of hospital admission (days). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified according to birthweight (< 1000 g and ≥ 1000 g) using a 2‐ and 4‐block design generated by randomised number tables. |
| Allocation concealment (selection bias) | Low risk | Allocation sealed in opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study milks indistinguishable in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessment blinded to trial group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Number of days of continuous positive airways pressure; number of days until completing the study; duration of parenteral feeding and human milk feeding in days; and daily milk intake in millilitres prespecified but not reported. |
Williams 2017.
| Methods | Randomised controlled trial Study sites: 21 neonatal units in the UK (2010–2012) |
|
| Participants | Newborn infants < 31 weeks' gestation at birth | |
| Interventions | Treatment (n = 637): sodium iodide given enterally or parenterally at 30 μg/kg/day Control (n = 636): sodium chloride given enterally or parenterally at 30 μg/kg/day Allocated trial solution continued until infants reached 34 weeks' gestational age |
|
| Outcomes | Primary: neurodevelopmental status defined by the Cognitive, Language, and Motor domains of the Bayley‐III at 2 years of age corrected for prematurity Secondary: *mortality, subtests of Bayley‐III; Bayley‐III analysed as a dichotomous outcome (death or Bayley‐III score < 85 in any of the main domains versus Bayley‐III score ≥ 85); plasma levels of T4, TSH, TBG up to 34–36 weeks' postmenstrual age; neonatal morbidity (RDS, CLD, PDA, NEC, hyperbilirubinaemia, acquired infections, cerebral pathology closest to 34 weeks', porencephalic cyst, cystic periventricular leukomalacia, ventriculopleural) |
|
| Notes | 1275 infants randomised. 2 infants randomised in error (n = 1273), 14 withdrawals (6 in iodine group, 8 in placebo group, no data used); sample size of 631 in iodine group and 628 in placebo group (n = 1259) *(61 infants in iodide group and 59 infants in placebo group died during the intervention stage of the trial, and 65 infants in iodide group and 66 infants in placebo group died before 2‐year follow‐up). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation programme using "bespoke minimization algorithm to ensure balance across hospitals on gender and gestational age." |
| Allocation concealment (selection bias) | Low risk | Allocation via "a secure website with telephone backup." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Packaging and visual appearance of trial solutions were identical." Research team, trial statistician, parents, neonatal staff, and pharmacy blinded to content of trial solutions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessment blinded to trial group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Days of endotracheal intubation and days of continuous positive airways pressure prespecified but not reported. |
Bayley‐III: Bayley Scales of Infant and Toddler Development, Third Edition; CLD: chronic lung disease; n: number of participants; NEC: necrotising enterocolitis; PDA: persistent ductus arteriosus; RDS: respiratory distress syndrome; T4: free thyroxine; TBG: thyroid‐binding globulin; TSH: thyroid‐stimulating hormone.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belykh 2014 | Reported as a conference abstract. Iodine given to mother during pregnancy and lactation. Likely that infant supplementation given via mother and iodine was not added to breast milk directly. |
| Bouhouch 2013 | Likely to be a trial restricted to term rather than preterm infants. Compared iodine supplementation for mothers versus iodine supplementation for infants (i.e. maternal supplementation was the control rather than no supplementation). |
| Kartoğlu 2014 | Gestation of participants not specified, and trial compared treatment of infants with congenital hypothyroidism. |
| Mohammed 2015 | Cluster randomised controlled trial of iodised salt given to pregnant women (rather than infants) in Ethiopia. |
| Wang 2013 | Chinese language report with English abstract. Likely to have been a cluster randomised controlled trial of effect of community‐based iodine supplementation for children up to 3 years old on neurodevelopment. |
Contributions of authors
VW and WM undertook the electronic and handsearches. VW and JVEB screened the title and abstract of all studies identified, and the full text of the report identified as of potential relevance.
VW and JVEB assessed the methodological quality of the included trials, and extracted the relevant information and data.
WM resolved disagreements.
All three authors completed the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
National Institute for Health Research (NIHR), UK.
This report is independent research funded by a UK NIHR Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the review authors and are not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
Declarations of interest
VW: none.
JVEB: none.
WG: none.
Edited (no change to conclusions)
References
References to studies included in this review
Rogahn 2000 {published data only}
- Rogahn J, Ryan S, Wells J, Fraser B, Squire C, Wild N, et al. Randomised trial of iodine intake and thyroid status in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2000;83(2):F86‐90. [PUBMED: 10952698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2017 {published data only}
- Williams F, Hume R, Ogston S, Brocklehurst P, Morgan K, Juszczak E, et al. A summary of the iodine supplementation study protocol (I2S2): a UK multicentre randomised controlled trial in preterm infants. Neonatology 2014;105(4):282‐9. [DOI: 10.1159/000358247; PUBMED: 24576827] [DOI] [PubMed] [Google Scholar]
- Williams FL, Ogston S, Hume R, Watson J, Stanbury K, Willatts P, et al. Supplemental iodide for preterm infants and developmental outcomes at 2 years: an RCT. Pediatrics 2017;139(5):e20163703. [DOI: 10.1542/peds.2016-3703; PUBMED: 28557747] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Belykh 2014 {published data only}
- Belykh N, Mamenko M, Kovalenko N, Minyaylo N, Plugatarenko N. Effectiveness of iodine prophylaxis in pregnant, lactating, mothers and infants in mild iodine deficiency region. European Thyroid Journal 2014;3 Suppl 1:117‐8. [Google Scholar]
Bouhouch 2013 {published data only}
- Bouhouch RR, Bouhouch S, Stinca S, Cherkaoui M, Aboussad A, Andersson M, et al. Infant iodine supplementation and motor and cognitive development: a randomized controlled trial. FASEB Journal 2013;27 Suppl 1:lb1‐1217.39. [Google Scholar]
Kartoğlu 2014 {published data only}
- Kurtoğlu S, Köroğlu Ş, Baştuğ O, Daar G, Yıkılmaz A, Elmalı F. The comparison of thyroxine versus thyroxine plus oral iodine in the treatment of congenital hypothyroidism due to iodine deficiency. Hormone Research in Paediatrics 2014;81(6):409‐15. [DOI: 10.1159/000358878; PUBMED: 24776962] [DOI] [PubMed] [Google Scholar]
Mohammed 2015 {published data only}
- Mohammed H, Marquis G, Aboud F, Bougma K, Samuel A. A cluster RCT evaluating the effect of iodized salt on infant development in Amhara region of Ethiopia. FASEB Journal 2015;29 Suppl 1:LB264. [Google Scholar]
Wang 2013 {published data only}
- Wang YL, Ge PF, Cao YQ, Zheng J, Dun W, Li HB, et al. Evaluation of iodine supplementation on improvement of developmental quotient at the critical period of infant brain development. Chinese Journal of Endemiology 2013;32(4):400‐3. [Google Scholar]
Additional references
Agostoni 2010
- Agostoni C, Buonocore G, Carnielli VP, Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85‐91. [DOI: 10.1097/MPG.0b013e3181adaee0; PUBMED: 19881390] [DOI] [PubMed] [Google Scholar]
Aitken 2014
- Aitken J, Williams FL. A systematic review of thyroid dysfunction in preterm neonates exposed to topical iodine. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(1):F21‐8. [DOI: 10.1136/archdischild-2013-303799; PUBMED: 24105624] [DOI] [PubMed] [Google Scholar]
Ares 1997
- Ares S, Escobar‐Morreale HF, Quero J, Duran S, Presas MJ, Herruzo R, et al. Neonatal hypothyroxinemia: effects of iodine intake and premature birth. Journal of Clinical Endocrinology and Metabolism 1997;82(6):1704‐12. [DOI: 10.1210/jcem.82.6.4019; PUBMED: 9177368] [DOI] [PubMed] [Google Scholar]
Belfort 2012
- Belfort MB, Pearce EN, Braverman LE, He X, Brown RS. Low iodine content in the diets of hospitalized preterm infants. Journal of Clinical Endocrinology and Metabolism 2012;97(4):E632‐6. [DOI: 10.1210/jc.2011-3369; PUBMED: 22337912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Delahunty 2010
- Delahunty C, Falconer S, Hume R, Jackson L, Midgley P, Mirfield M, et al. Levels of neonatal thyroid hormone in preterm infants and neurodevelopmental outcome at 5 1/2 years: millennium cohort study. Journal of Clinical Endocrinology and Metabolism 2010;95(11):4898‐908. [DOI: 10.1210/jc.2010-0743; PUBMED: 20719832] [DOI] [PubMed] [Google Scholar]
Den Ouden 1996
- Ouden AL, Kok JH, Verkerk PH, Brand R, Verloove‐Vanhorick SP. The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatric Research 1996;39:142‐5. [DOI: 10.1203/00006450-199601000-00021] [DOI] [PubMed] [Google Scholar]
Forhead 2014
- Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. Journal of Endocrinology 2014;221(3):R87‐103. [DOI: 10.1530/JOE-14-0025; PUBMED: 24648121] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 15 March 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2017
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Hollanders 2015
- Hollanders JJ, Israels J, Pal SM, Verkerk PH, Rotteveel J, Finken MJ. No association between transient hypothyroxinemia of prematurity and neurodevelopmental outcome in young adulthood. Journal of Clinical Endocrinology and Metabolism 2015;100(12):4648‐53. [DOI: 10.1210/jc.2015-3078; PUBMED: 26480285] [DOI] [PubMed] [Google Scholar]
Hollanders 2016
- Hollanders JJ, Pal SM, Verkerk PH, Rotteveel J, Finken MJ. Transient hypothyroxinemia of prematurity and problem behavior in young adulthood. Psychoneuroendocrinology 2016;72:40‐6. [DOI: 10.1016/j.psyneuen.2016.06.008; PUBMED: 27343725] [DOI] [PubMed] [Google Scholar]
Ibrahim 2003
- Ibrahim M, Morreale de Escobar GM, Visser TJ, Duran S, Toor H, Strachan J, et al. Iodine deficiency associated with parenteral nutrition in extreme preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(1):F56‐7. [PUBMED: PMC1756012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kieran 2018
- Kieran EA, O'Sullivan A, Miletin J, Twomey AR, Knowles SJ, O'Donnell CP. 2% chlorhexidine‐70% isopropyl alcohol versus 10% povidone‐iodine for insertion site cleaning before central line insertion in preterm infants: a randomised trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(2):F101‐6. [DOI: 10.1136/archdischild-2016-312193; PUBMED: 29074717] [DOI] [PubMed] [Google Scholar]
Koo 2017
- Koo W, Tice H. Human milk fortifiers do not meet the current recommendation for nutrients in very low birth weight infants. Journal of Parenteral and Enteral Nutrition 2017;42(4):813‐20. [DOI: 10.1177/0148607117713202; PUBMED: 28622483] [DOI] [PubMed] [Google Scholar]
Liggins 1988
- Liggins GC, Schellenberg JC, Manzai M, Kitterman JA, Lee CC. Synergism of cortisol and thyrotropin releasing hormone in lung maturation in fetal sheep. Journal of Applied Physiology 1988;65(4):1880‐4. [DOI: 10.1152/jappl.1988.65.4.1880; PUBMED: 3141365] [DOI] [PubMed] [Google Scholar]
Lucas 1996
- Lucas A, Morley R, Fewtrell MS. Low triiodothyronine concentration in preterm infants and subsequent intelligence quotient (IQ) at 8 year follow up. BMJ 1996;312(7039):1132‐3. [PUBMED: 8620130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meijer 1992
- Meijer WJ, Verloove‐Vanhorick SP, Brand R, Brande JL. Transient hypothyroxinaemia associated with developmental delay in very preterm infants. Archives of Disease in Childhood 1992;67(7):944‐7. [PUBMED: 1381573] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2004
- Murphy N, Hume R, Toor H, Matthews TG, Ogston SA, Wu SY, et al. The hypothalamic‐pituitary‐thyroid axis in preterm infants; changes in the first 24 hours of postnatal life. Journal of Clinical Endocrinology and Metabolism 2004;89(6):2824‐31. [DOI: 10.1210/jc.2003-030317; PUBMED: 15181064] [DOI] [PubMed] [Google Scholar]
Osborn 2007a
- Osborn DA, Hunt RW. Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005948.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2007b
- Osborn DA, Hunt RW. Postnatal thyroid hormones for preterm infants with transient hypothyroxinaemia. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005945.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reuss 1996
- Reuss ML, Paneth N, Pinto‐Martin JA, Lorenz JM, Susser M. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. New England Journal of Medicine 1996;334(13):821‐7. [DOI: 10.1056/NEJM199603283341303; PUBMED: 8596548] [DOI] [PubMed] [Google Scholar]
Reuss 1997
- Reuss ML, Leviton A, Paneth N, Susser M. Thyroxine values from newborn screening of 919 infants born before 29 weeks' gestation. American Journal of Public Health 1997;87(10):1693‐7. [PUBMED: 9357357] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rooman 1996
- Rooman RP, Du Caju MV, Beeck LO, Docx M, Reempts P, Acker KJ. Low thyroxinaemia occurs in the majority of preterm newborns. European Journal of Pediatrics 1996;155(3):211‐5. [PUBMED: 8929730] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Scratch 2014
- Scratch SE, Hunt RW, Thompson DK, Ahmadzai ZM, Doyle LW, Inder TE, et al. Free thyroxine levels after very preterm birth and neurodevelopmental outcomes at age 7 years. Pediatrics 2014;133(4):e955‐63. [DOI: 10.1542/peds.2013-2425; PUBMED: 24685955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smerdely 1989
- Smerdely P, Lim A, Boyages SC, Waite K, Wu D, Roberts V, et al. Topical iodine containing antiseptics and neonatal hypothyroidism in very‐low‐birthweight infants. Lancet 1989;2(8664):661‐4. [PUBMED: 2570908] [DOI] [PubMed] [Google Scholar]
van Wassenaer‐Leemhuis 2014
- Wassenaer‐Leemhuis A, Ares S, Golombek S, Kok J, Paneth N, Kase J, et al. Thyroid hormone supplementation in preterm infants born before 28 weeks' gestational age and neurodevelopmental outcome at age 36 months. Thyroid 2014;24(7):1162‐9. [DOI: 10.1089/thy.2013.0618; PUBMED: 24684245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Velasco 2018
- Velasco I, Bath SC, Rayman MP. Iodine as essential nutrient during the first 1000 days of life. Nutrients 2018;10(3):E290. [DOI: 10.3390/nu10030290; PUBMED: 29494508] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2008
- Williams FL, Hume R. Perinatal factors affecting thyroid hormone status in extreme preterm infants. Seminars in perinatology 2008;32(6):398‐402. [DOI: 10.1053/j.semperi.2008.09.004; PUBMED: 19007676] [DOI] [PubMed] [Google Scholar]
Williams 2011
- Williams F, Hume R. The measurement, definition, aetiology and clinical consequences of neonatal transient hypothyroxinaemia. Annals of Clinical Biochemistry 2011;48(Pt 1):7‐22. [PUBMED: 20930033] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ibrahim 2006
- Ibrahim M, Sinn J, McGuire W. Iodine supplementation for the prevention of mortality and adverse neurodevelopmental outcomes in preterm infants. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005253.pub2] [DOI] [PubMed] [Google Scholar]


