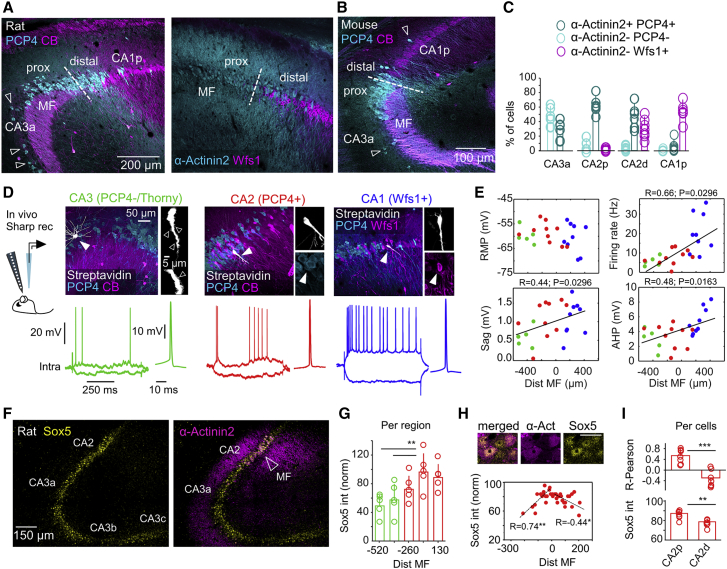
Figure 2.
Cell-Type-Specific Heterogeneity around CA2
(A) Immunoreactivity against PCP4, α-Actinin2, CB, and Wfs1 allowed evaluating cell-type heterogeneity around CA2. Images show co-localization among different markers (3 confocal optical sections). Some PCP4+ cells were identified in deep layers of CA3a and in CA1 (open arrows). MF was used to define the proximal (close to CA3a) and distal (close to CA1) sectors of CA2.
(B) PCP4 and CB expression in the mouse CA2.
(C) Quantification of pyramidal cell types around CA2, as examined in double immunostaining against α-Actinin2/PCP4 (n = 6 sections from 3 rats) and α-Actinin2/Wfs1 (n = 6 sections from 3 rats). Individual data points are shown, together with mean ± SD.
(D) Intracellular recordings of CA2 pyramidal cells obtained in vivo from urethane anesthetized rats. Cells with thorny excrescences (open arrowheads) and lacking immunoreactivity for PCP4 or α-Actinin2 were classified as CA3 (green, n = 5). Cells positive to Wfs1 either CB+ or CB− were classified as CA1 (blue, n = 9). Cells immunoreactive to PCP4 or α-Actinin2 without thorny excrescences were classified as CA2 cells (red, n = 10).
(E) Intrinsic properties of the different cell types plotted as a function of their distance to MF. Proximodistal gradients were confirmed by a Pearson correlation, as indicated. Intrinsic properties were measured at the resting membrane potential (RMP). Sag and firing rate were calculated in response to ±0.3 nA current pulses. AHP was calculated from the first spike in response to +0.2 nA.
(F) Immunohistochemical expression of Sox5 in a representative section of the rat dorsal hippocampus co-localized with α-Actinin2 (1 confocal section).
(G) Quantification of Sox5 expression per region (normalized by background at 0) along the proximodistal axis of CA3 to CA2 (n = 5 sections from 4 rats). Significant one-way ANOVA, F(24) = 4.7, p = 0.008. Post hoc Tukey test, ∗∗p < 0.001. Error bars show mean ± SD.
(H) Expression of Sox5 in CA2 cells as a function of the cell distance to MF (1 confocal section). A Pearson correlation R index was evaluated for proximal (−250 to 0 μm) and distal (0 to 250 μm) sectors separately. ∗p < 0.05, ∗∗p < 0.001. One representative section is shown.
(I) Mean group data (±SD) of the Pearson correlation and mean normalized intensity for proximal and distal CA2 cells. Data from n = 7 sections from 4 rats. Paired Student’s (two-tailed) t tests, ∗∗p < 0.01, ∗∗∗p < 0.0001.