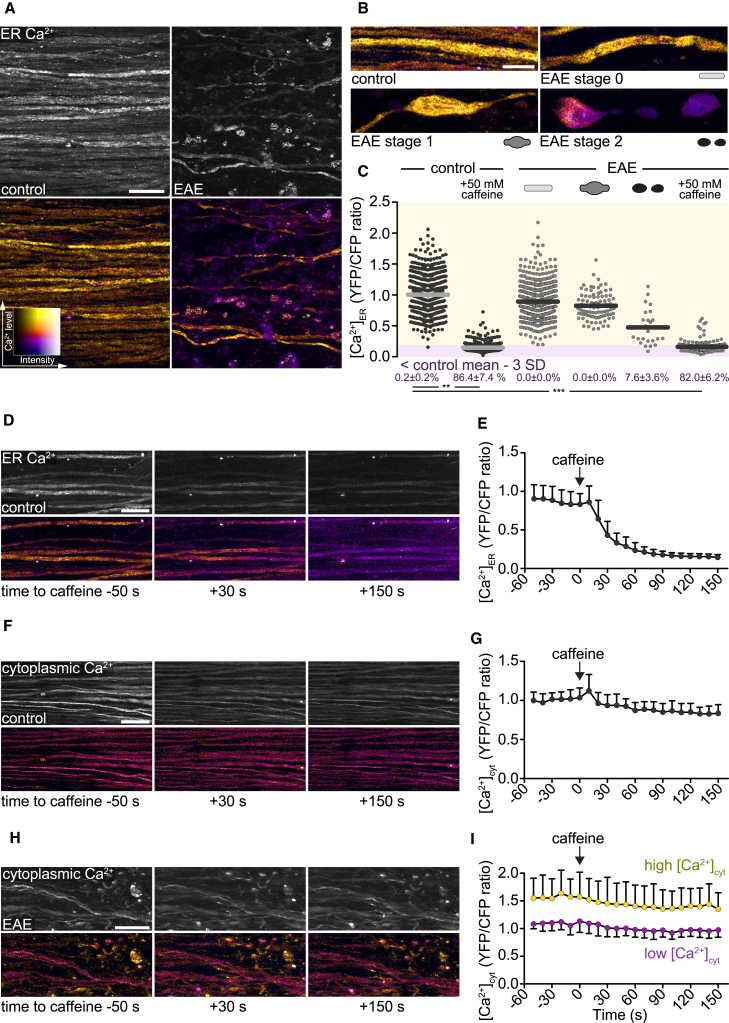
Figure 2.
Ca2+ Release from Axoplasmic Reticulum Does Not Cause Sustained Changes of Cytoplasmic Ca2+ Levels in Healthy and EAE Axons
(A) In vivo multiphoton maximum intensity projection of spinal cord axons of healthy (left) and EAE (2 days after onset, right) Thy1-TwitchER mice. Top: grayscale images of YFP channel. Bottom: ratiometric (YFP/CFP) images color coded for ER calcium levels ([Ca2+]ER).
(B) Ratiometric projection images showing [Ca2+]ER in healthy axons and normal-appearing (stage 0), swollen (stage 1), and fragmented (stage 2) axons in acute EAE lesions.
(C) [Ca2+]ER of single axons (YFP/CFP channel ratio normalized to the mean of control axons) in healthy spinal cord and different focal axonal degeneration stages in EAE (before and after application of 50 mM caffeine, respectively). Percentages (below) show the proportion of axons with depleted [Ca2+]ER < control mean – 3 SD, shown as mean ± SEM (tested per animal in n = 7 control and n = 7 EAE mice, paired t test control versus caffeine, Mann-Whitney U test control versus EAE stages 0–2 and EAE + caffeine, respectively).
(D, F, and H) Time-lapse projection images of axons in control spinal cord (D and F) and in an EAE lesion (H) before and after application of 50 mM caffeine. [Ca2+]ER is color coded in (D) and [Ca2+]cyt is color coded in (F) and (H).
(E, G, and I) Time course of control axon-normalized [Ca2+]ER (E) and [Ca2+]cyt (G and I) before and after caffeine application. Purple circles in (I) indicate low and yellow circles high [Ca2+]cyt axons at time point −50 s.
Presented as mean ± SD (n = 26 axons in E, 46 axons in G, 23 low versus 17 high [Ca2+]cyt axons in I). Scale bars in (A), (D), (F), and (H), 25 μm, and in (B), 10 μm. ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S2 and Video S1.