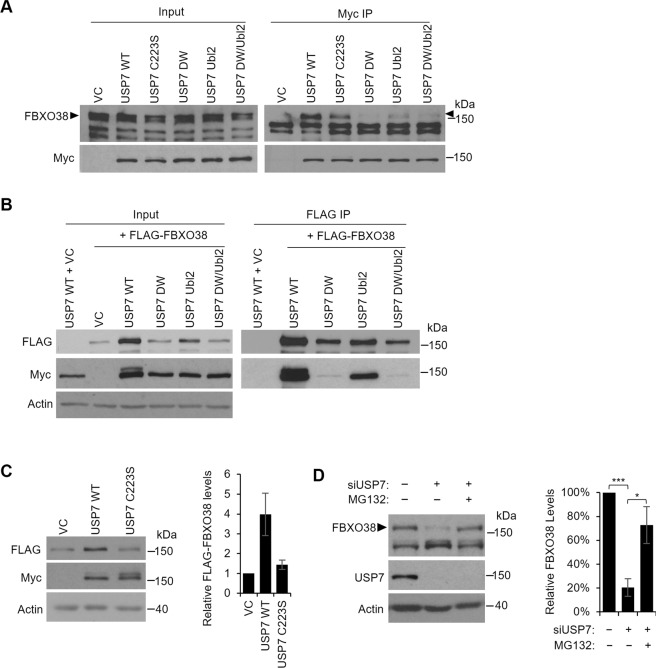
Figure 1.
USP7 interacts with and stabilizes FBXO38. (A) AGS cells were transfected with a plasmid expressing myc-tagged USP7 WT, the USP7 catalytic mutant (C223S), the USP7 NTD pocket mutant (DW), the USP7 Ubl2 pocket mutant (Ubl2), the USP7 double pocket mutant (DW/Ubl2) or an empty vector control (VC). Myc-USP7 constructs were immunoprecipitated with anti-myc antibody and recovered proteins were analyzed by Western blotting using antibodies against myc and endogenous FBXO38. The band corresponding to FBXO38 is indicated with arrow heads. (B) HEK293T cells were transfected with a plasmid expressing myc-tagged USP7 WT or co-transfected with plasmids expressing FLAG-tagged FBXO38 and each of the indicated myc-tagged USP7 plasmids. FBXO38 was immunoprecipitated using anti-FLAG M2 resin and recovered proteins were analyzed by Western blotting using antibodies against FLAG and myc. (C) HEK293T cells were co-transfected with plasmids expressing FLAG-tagged FBXO38 and either myc-USP7 WT, C223S or an empty vector control (VC). Cells were harvested 48 h post transfection and cell lysates were analyzed by Western blotting using antibodies against FLAG, Myc and actin. Quantification of the FLAG-FBXO38 bands (normalized to actin) from two independent experiments is shown on the right. (D) AGS cells were transfected with an siRNA targeting USP7 (+) or a negative control siRNA (−) followed by treatment with the MG132 proteasome inhibitor (+) or DMSO as a negative control (−) for 12 hours. Cell lysates were analyzed by Western blotting using antibodies against FBXO38, USP7 and actin. Quantification of the FBXO38 bands (normalized to actin) from three independent USP7 silencing experiments are shown on the right. ***P < 0.001.