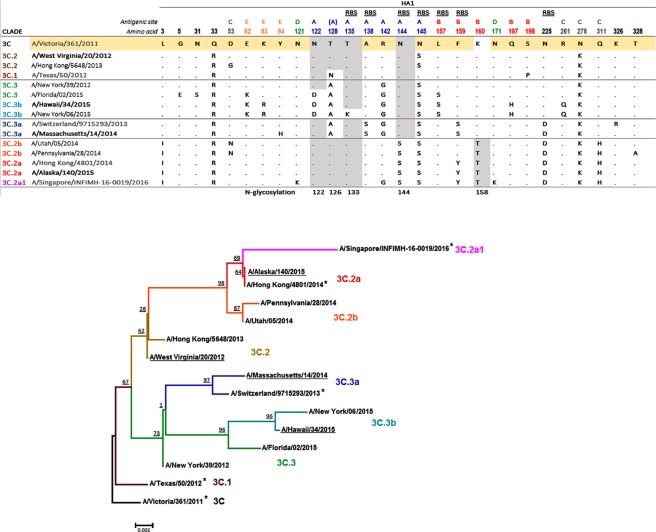
Figure 1.
Representative A(H3N2) viruses utilized to generate HINT reference material. (A) HA1 sequences of human respiratory specimens selected to produce reference material (shown in bold) and cell-grown counterparts of viruses recommended as A(H3N2) vaccine strains during 2012–2017 (shown in grey). A/Victoria/361/2011 (M1: EPI ISL 101506), A/Texas/50/2012 (M1,C1: EPI ISL 101506), A/Switzerland/9715293/2013 (S1: EPI ISL 162149), A/Hong Kong/4801/2014 (S1: EPI ISL 165554); A/Singapore/INFIMH-16-0019/2016 (original: EPI ISL 225834) was recommended for the SH 2018 season. All viruses listed share sequons of N-glycosylation at residues 8, 22, 38, 45, 63, 165, 246, 246, 285 in HA1 and 154 in HA2. AA 128 belong to antigenic site B, however substitution T128N/A results in loss of glycosylation on N126 (antigenic site A). RBS: Receptor binding site. (B) Phylogenetic relationship among representative influenza A(H3N2) HA genes. Clinical specimens used in this study are underlined, and vaccines are marked with an asterisk. Branching patterns are color-coded by A(H3N2) HA genetic clade. Bootstraps per parent node and the scale bar (in nucleotide mutations per site) are indicated.