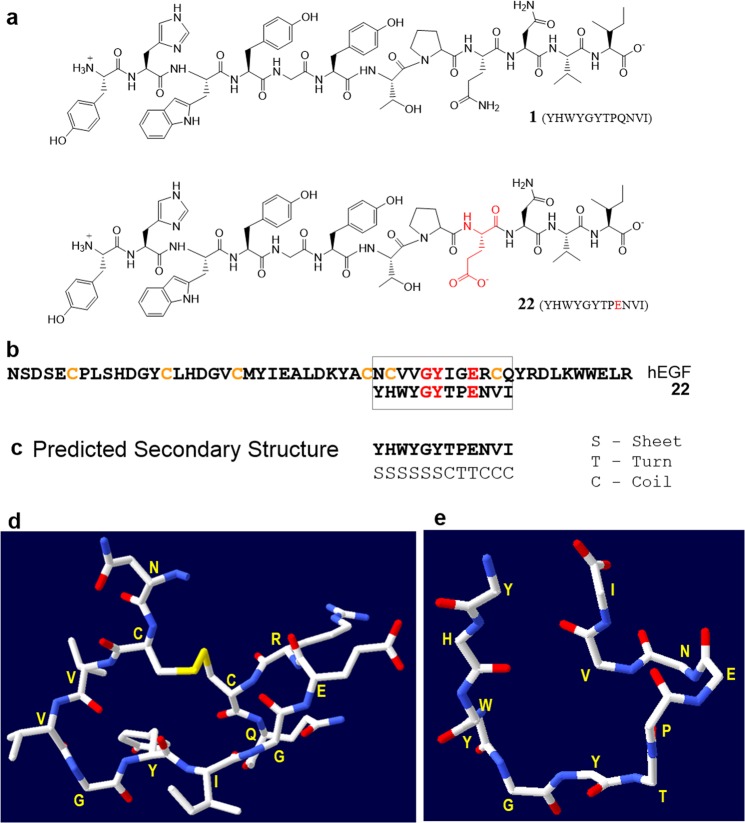
Figure 4.
(a) Chemical structure of peptide 1 (GE11) and peptide analogue 22. (b) Amino acid sequence of 53-mer human EGF (hEGF) and 12-mer 22 showing sequence homology for the G, Y and E residues (highlighted in red). Peptide 22 also shows structural similarity (based on secondary structure prediction of 22) to this cyclic region of hEGF (residues 32–43). (c) Predicted secondary structure of peptide 22 using Chou and Fasman secondary structure prediction (CFSSP) server37,38. (d) 3D structure of a portion (residues 32–43) of hEGF derived from the crystal structure of full length hEGF (PDB code 1JL9)35. (e) Energy minimized structure of peptide 22 obtained using PEP-fold 2.043 and Swiss-Pdb Viewer 4.142. Side chains are not shown here for clarity.