Abstract
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited disease that exhibits sex differences on clinical presentation. The present study aimed to investigate the sex differences associated with ARVC by conducting an integrated bioinformatics analysis. The GSE29819 gene expression dataset was downloaded from the Gene Expression Omnibus database. The online analytical tool GEO2R was then used to screen for differentially expressed genes (DEGs), which were subsequently processed using enrichment analysis and protein-protein interaction (PPI) network construction. Functional annotation of the DEGs was determined using ClueGO. The PPI network was constructed with Search Tool for the Retrieval of Interacting Genes, and was visualized with Cytoscape to identify the modules and hub genes. Compared with the female group, a total of 1,188 DEGs, of which 915 were upregulated and 273 were downregulated, were identified in the male group. The enrichment analysis revealed that in KEGG pathways, the upregulated DEGs were substantially enriched in the ‘nicotine addiction’ pathways, whereas the downregulated DEGS were mainly enriched in the ‘ECM-receptor interaction’ and ‘protein digestion and absorption’ pathways. The PPI network contained 899 nodes and 1,627 edges, among which four significant modules were identified. In addition, kininogen 1, lysophosphatidic acid receptor 5, formyl peptide receptor (FPR) 2, adenylate cyclase 2, γ-aminobutyric acid type B receptor subunit 2, FPR1, hydroxycarboxylic acid receptor 1, prostaglandin E receptor 3, cannabinoid receptor 1 and proenkephalin were identified as the top 10 hub genes. The key genes and related pathways identified in this study provide genetic insight into the diversity in phenotypes between female and male patients with ARVC, and may facilitate therapeutic individualization.
Keywords: arrhythmogenic right ventricular cardiomyopathy, integrated bioinformatics analysis, ventricular tachycardia, sudden cardiac death
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an autosomal dominant inherited disease. The distinguishing feature of ARVC is the replacement of the myocardium with fibrous fatty tissue, which leads to ventricular tachycardia (VT) and ultimately results in sudden cardiac death (SCD) (1). As reported in previous studies, the majority of patients with ARVC are male (2–5). In addition, the sex disparity in phenotypic presentations of ARVC has been demonstrated by numerous studies. Naneix et al (6) indicated that the cause of SCD was more often the cardiomyopathy in women, particularly arrythmogenic right ventricular cardiomyopathy (ARVC). In life-threatening arrhythmias, numerous studies have demonstrated that the male sex is associated with a significantly higher risk of VT (7–9). In order to provide individualized therapy for patients with ARVC, the present study aimed to identify key genes and pathways that may be associated with the diversity of phenotypes between female and male patients.
In the present study, the GSE29819 gene expression dataset was downloaded from the Gene Expression Omnibus (GEO) database. Differentially expressed genes (DEGs) were defined as genes with significantly different expression levels between female and male patients with ARVC. The biological functions of the DEGs were identified using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses (10,11). The protein-protein interaction (PPI) network was constructed using the Search Tool for the Retrieval of Interacting Genes (STRING) database, in order to detect the hub genes and modules (12). The results of the present bioinformatics analysis may aid elucidation of the sex differences of ARVC by identifying the key genes and pathways that contribute to the phenotypes.
Materials and methods
Microarray data
The GSE29819 gene expression dataset (13) is based on the gene chip of the Human Genome U133 Plus 2.0 Array platform (Affymetrix; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and was retrieved from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). The GSE29819 dataset contains 38 ventricular myocardial samples, including 12 tissues from patients with ARVC, 14 tissues from patients with dilated cardiomyopathy and 12 tissues from individuals with non-failing hearts. The 12 tissues from patients with ARVC were divided into two groups according to sex: The female patients group (4 samples) and the male patients group (8 samples). The identification of DEGs was conducted using GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/), which is an online analytical tool for performing comparisons on raw data with the limma R package and GEO-query (14). The results of the comparisons were extracted in file format, and the cut-off criteria for DEGs were set as follows: P<0.05 and |log fold-change|>1.5.
GO and KEGG enrichment analyses
GO and KEGG enrichment analyses of the upregulated and downregulated DEGs were conducted separately with the Cytoscape plugin ClueGO (version 2.5.2) (15). The criteria for statistically significant differences were set as follows: P<0.01 and a κ score of 1.0.
Integration of the PPI network
Interactions among the DEGs were evaluated using the STRING (version 10.5) database; a combined score of >0.4 was considered to indicate a statistically significant interaction (16). The integrated networks were visualized with Cytoscape software (version 3.6.1) (17). In addition, the Cytoscape plugin Molecular Complex Detection (MCODE; version 1.31), with a cutoff criterion of MCODE score >5, was used to identify molecular modules in the PPI network (18). Further functional annotation of the modules was achieved with STRING (version 10.5) (16). The Cytoscape plugin cytoHubba (version 0.1), with the ranking methods of maximal clique centrality (MCC), was used to identify the hub genes (19).
Results
Identification of DEGs in patients with ARVC of different sexes
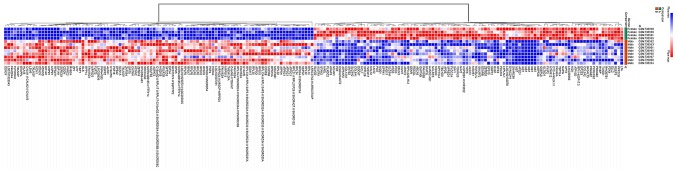
The datasets were analyzed using GEO2R and 1,188 DEGs were identified, of which 915 were upregulated and 273 were downregulated in the male group. The DEGs were used to construct a heat map (Fig. 1) and a volcano plot (Fig. 2).
Figure 1.
Heat map of the top 100 differentially expressed genes. Red indicates upregulated genes and blue indicates downregulated genes.
Figure 2.
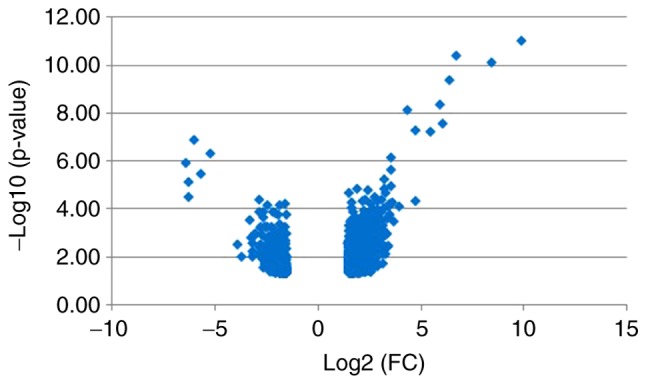
Volcano plot of DEGs. Blue indicates DEGs with an absolute value of |log2FC|>1.5. DEGs, differentially expressed genes; FC, fold change.
Functional enrichment analysis
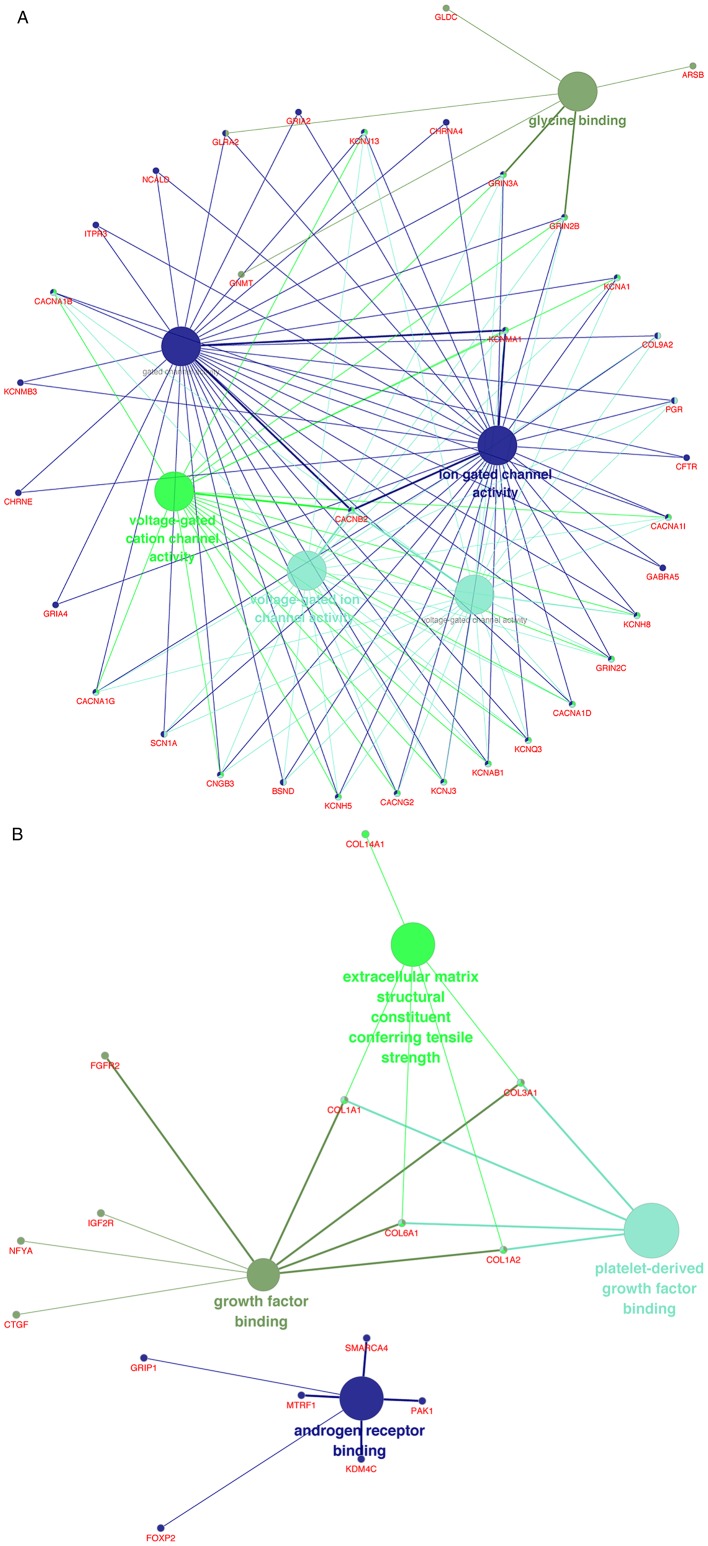
The upregulated and downregulated DEGs were processed separately with ClueGO for GO and KEGG pathway analyses. In terms of molecular function (MF), the upregulated DEGs were mostly enriched in ‘glycine binding’, ‘voltage-gated cation channel activity’, ‘voltage-gated ion channel activity’, ‘voltage-gated channel activity’, ‘gated channel activity’ and ‘ion gated channel activity’ (Fig. 3A), whereas the downregulated DEGs were mainly enriched in ‘growth factor binding’, ‘extracellular matrix structural constituent conferring tensile strength’, ‘platelet-derived growth factor binding’ and ‘androgen receptor binding’ (Fig. 3B).
Figure 3.
(A) Enrichment of upregulated DEGs in MF terms. (B) Enrichment of downregulated DEGs in MF terms. DEGs, differentially expressed genes; MF, molecular function.
The KEGG pathway analysis revealed that the upregulated DEGs were substantially enriched in the ‘nicotine’ pathways (Fig. 4A), whereas the downregulated DEGs were substantially enriched in the ‘ECM-receptor interaction’ and ‘protein digestion and absorption’ pathways (Fig. 4B).
Figure 4.
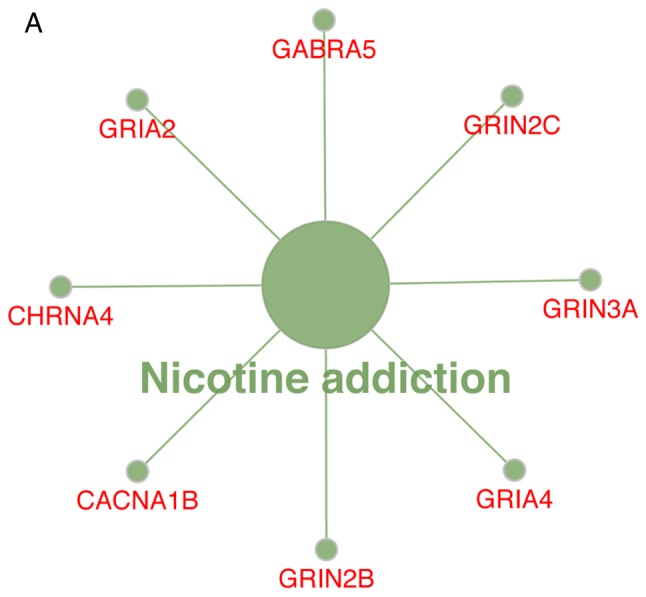
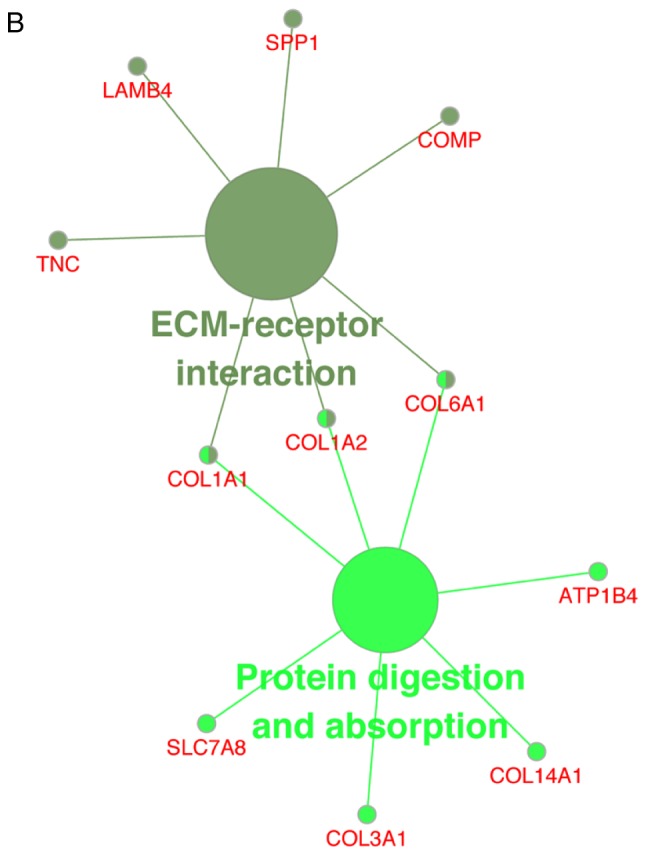
(A) Enrichment of upregulated DEGs in KEGG pathways. (B) Enrichment of downregulated DEGs in KEGG pathways. DEGs, differentially expressed genes; KEGG, Kyoto Encyclopedia of Genes and Genomes.
PPI network construction, and module and hub gene identification
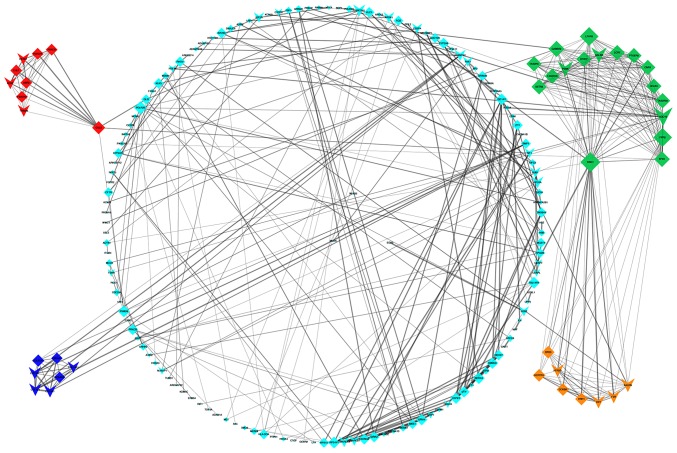
Detection of interactions among the DEGs was performed by constructing a PPI network, which contained 899 nodes and 1,627 edges (Fig. 5). With a cutoff criterion of MCODE score >5, four modules were identified, which were significantly enriched in the following pathways: DEGs in module 1, including LPAR5, FPR2, ADCY2, GABBR2, FPR1, HCAR1, PTGER3 and CNR1, were enriched in ‘neuroactive ligand-receptor interaction’, ‘taste transduction’ and ‘Rap1 signaling pathway’; DEGs in module 2, including KLHL25, CUL3, HERC1, CUL7, CCNF, UBR2, FBXO9, FBXO10 and TRIM36, were enriched in ‘ubiquitin mediated proteolysis’; DEGs in module 3, including OXT, HRH1, F2R, KALRN, CCKBR, HTR2B, HCRTR1 and BRS3, were enriched in ‘neuroactive ligand-receptor interaction’ and ‘calcium signaling pathway’; and DEGs in module 4, including COL1A2, COL1A1, COL3A1, COL6A1, COL14A1, COL27A1 and COL9A2, were enriched in ‘protein digestion and absorption’, ‘ECM-receptor interaction’, ‘focal adhesion’, ‘PI3K-Akt signaling pathway’, ‘amoebiasis’ and ‘platelet activation’ (Fig. 6). Based on the ranking methods of MCC, the following genes were identified as the top 10 hub genes (Fig. 7): Kininogen 1 (KNG1), lysophosphatidic acid receptor 5 (LPAR5), formyl peptide receptor (FPR) 2, adenylate cyclase 2 (ADCY2), γ-aminobutyric acid type B receptor subunit 2 (GABBR2), FPR1, hydroxycarboxylic acid receptor 1 (HCAR1), prostaglandin E receptor 3 (PTGER3), cannabinoid receptor 1 (CNR1) and proenkephalin (PENK).
Figure 5.
Protein-protein interaction network; Green, module 1; red, module 2; yellow, module 3; blue, module 4. Arrowheads indicate upregulated DEGs and diamonds indicate downregulated DEGs. DEGs, differentially expressed genes.
Figure 6.
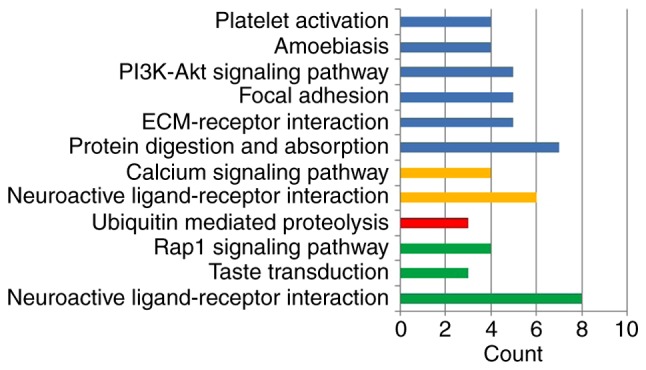
Enrichment of modules in Kyoto Encyclopedia of Genes and Genomes pathways. Green, module 1; red, module 2; yellow, module 3; blue, module 4.
Figure 7.
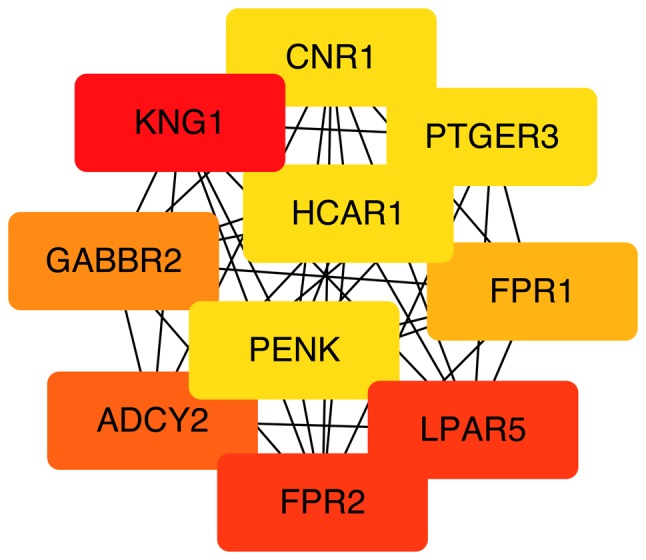
Top 10 hub genes. Color depth for ranking of hub genes. The sequence of colors is red-orange-yellow from high ranking to low ranking.
Discussion
Numerous studies have focused on the sex differences in the phenotypes of ARVC and have revealed substantial diversity in clinical presentations, including SCD or VT, between female and male patients (6–9). The aim of the present study was to aid identification of the cause of these differences using bioinformatics analysis.
ARVC is an inherited disease with 12 confirmed related genes. Notably, none of these 12 genes exhibited statistical sex differences in the present study, indicating the possible involvement of other symptom-associated genes. In the present study, the genetic profile data were extracted from the GEO database and analyzed with GEO2R. The analysis revealed that 1,188 genes were differentially expressed between the female and male patients with ARVC, of which 273 were downregulated and 915 were upregulated.
A previous study identified a splice site homozygous deletion in solute carrier family 6 member 6 (SLC6A6) in patients with idiopathic dilated cardiomyopathy, indicating the possible role of SLC6A6 in the pathophysiology of heart failure (HF) (20). Natriuretic peptide (NPP) A and NPPB were two significantly downregulated DEGs in the present study. The NPPB promoter regulates the dynamic expression of NPPA, and NPPA has previously been demonstrated to be highly expressed during ventricular stress (21). The disparity detected in NPPA expression suggests markedly different levels of ventricular stress between the groups, which may leads to diverse phenotypes. Ubiquitin specific peptidase 9 Y-linked (USP9Y), ribosomal protein S4 Y-linked 1 (RPS4Y1) and eukaryotic translation initiation factor 1A Y-linked (EIF1AY) were significantly upregulated DEGs in the present study. Similar patterns of expression were demonstrated by Heidecker et al (22), thus suggesting that overexpression of USP9Y, RPS4Y1 and EIF1AY may contribute to sex differences in the pathophysiology of HF.
The GO analysis revealed that, in the molecular function domain, the downregulated DEGs were significantly enriched in extracellular matrix (ECM) structural constituent. A previous study demonstrated that proteolysis-mediated signaling can enhance accumulation of the ECM, which contributes to negative remodeling of the left ventricle and may eventually lead to severe HF (23). This may be a possible mechanism in certain patients that present with severe HF. In the MF domain, the majority of DEGs in the present study were enriched in ‘glycine binding’, ‘voltage-gated cation channel activity’, ‘voltage-gated ion channel activity’, ‘growth factor binding’, ‘extracellular matrix structural constituent conferring tensile strength’, ‘platelet-derived growth factor binding’ and ‘androgen receptor binding’. A number of these results are consistent with those of previous studies. Qi et al (24) suggested that glycine protects cardiomyocytes from lipopolysaccharide and hypoxia/reoxygenation injury by blocking the influx of calcium. Numerous studies have demonstrated that ‘voltage-gated channel’ markedly contributes to VT (25–27). Therefore, dysregulation in these DEGs may cause different phenotypes between the sexes.
Using MCODE, four modules were identified that were enriched in the following pathways: ‘neuroactive ligand-receptor interaction’, ‘taste transduction’, ‘Rap1 signaling pathway’, ‘ubiquitin mediated proteolysis’, ‘calcium signaling pathway’, ‘protein digestion and absorption’, ‘ECM-receptor interaction’, ‘focal adhesion’, ‘PI3K-Akt signaling pathway’, ‘amoebiasis’ and ‘platelet activation’. These pathways may have served a role in the diversity of clinical presentations between the sexes. Using the KEGG database (28), the majority of pathways enriched in modules 1 and 3 were mapped in ‘environmental information processing’, including ‘signaling molecules and interaction’ and ‘Signal transduction’. The DEGs in module 1, including LPAR5, FPR2, ADCY2, GABBR2, FPR1, HCAR1, PTGER3 and CNR1, were involved in the ‘signaling molecules and interaction’ and ‘signal transduction’ pathways, suggesting that these hubs contribute to the sex differences of phenotypes via ‘environmental information processing’. Pathway of ‘ubiquitin mediated proteolysis’ was enriched in module 2, indicating the possible involvement of the well-known protein degradation systems ‘ubiquitin proteasome’ in the development of ARVC (29). In addition to ‘environmental information processing’, the pathways of ‘amoebiasis’, ‘platelet activation’ and ‘protein digestion and absorption’ were significantly enriched in module 4. Since both the ‘amoebiasis’ and ‘platelet activation’ pathways participate in the activity of the immune system, it may be hypothesized that the DEGs (collagen type I α1 chain, collagen type I α2 chain, collagen type XXVII α1 chain and collagen type III α1 chain) in module 4 function in the various clinical presentations of ARVC via the immune mechanism (30,31).
The following 10 hub genes with a high level of connectivity were identified using cytoHubba: KNG1, LPAR5, FPR2, ADCY2, GABBR2, FPR1, HCAR1, PTGER3, CNR1 and PENK. Variations of the KNG gene can impact the aldosterone response, which markedly contributes to structural alterations in the heart (32,33). FPR agonists act in favor of cardioprotection (34). Therefore upregulation of the DEGs KNG1, FPR1 and FPR2 in female patients may reduce pathological cardiac reconstruction and increase cardiac protection, resulting in a lower chance of SCD. A similar deduction can be made for LPAR5, which is expressed in H9c2 cardiomyocytes. Ectonucleotide pyrophosphatase/phosphodiesterase 2/LPAR5 signaling regulates ferroptosis-related genes (glutathione peroxidase 4, acyl-CoA synthetase long chain family member 4 and nuclear factor, erythroid 2 like 2) and adjusts the survival signals of cardiomyocytes in order to protect cardiomyocytes from ferroptosis. PENK encodes the protein proenkephalin, which is mainly located in skeletal and cardiac muscle (35). In a previous study, the level of PENK was used to assess the progression of HF (36). Treskatsch et al (37) demonstrated that rats with severe HF develop overexpression of PENK. Furthermore, PENK is considered an independent risk predictor in the prognosis of patients with HF (38). Therefore, downregulation of PENK in female patients indicated that relatively preserved heart function may be the reason for a lower incidence of SCD. A limitation of the present study is that verification was not conducted, as ARVC is a relatively rare disease and the number of available genetic data profiles is limited.
The present study used bioinformatics analysis as a tool to identify genes and pathways that may be responsible for the sex differences in ARVC. The results of this study provided genetic insights into the diversity in the phenotype and clinical characteristics between female and male patients. However, further studies that verify these results are required. Only a few of the candidate DEGs identified in this study have been associated with HF or VT in previous studies. Further studies are required to establish the specific molecular mechanisms underlying the effects of the DEGs and pathways involved in different clinical presentations of ARVC between the sexes.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- GEO
Gene Expression Omnibus
- DEGs
differentially expressed genes
- PPI
protein-protein interaction
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- VT
ventricular tachycardia
- SCD
sudden cardiac death
- MF
molecular function
- MCC
maximal clique centrality
- ECM
extracellular matrix
- HF
heart failure
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the Gene Expression Omnibus repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE29819).
Authors' contributions
LTC analyzed and interpreted the data, and wrote the manuscript. CYJ made substantial contributions to the conception and study design, revised the manuscript critically for important intellectual content and gave the final approval of the version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Maupain C, Badenco N, Pousset F, Waintraub X, Duthoit G, Chastre T, Himbert C, Hébert JL, Frank R, Hidden-Lucet F, Gandjbakhch E. Risk stratification in arrhythmogenic right ventricular cardiomyopathy/dysplasia without an implantable cardioverter-defibrillator. JACC Clin Electrophysiol. 2018;4:757–768. doi: 10.1016/j.jacep.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 3.Beffagna G, Occhi G, Nava A, Vitiello L, Ditadi A, Basso C, Bauce B, Carraro G, Thiene G, Towbin JA, et al. Regulatory mutations in transforming growth factor-beta3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc Res. 2005;65:366–373. doi: 10.1016/j.cardiores.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–1179. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 5.Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, Sen Chowdhry S, McKenna WJ. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet. 2006;79:978–984. doi: 10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naneix AL, Périer MC, Beganton F, Jouven X, Lorin de la Grandmaison G. Sudden adult death: An autopsy series of 534 cases with gender and control comparison. J Forensic Leg Med. 2015;32:10–15. doi: 10.1016/j.jflm.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Kimura Y, Noda T, Otsuka Y, Wada M, Nakajima I, Ishibashi K, Miyamoto K, Okamura H, Aiba T, Kamakura S, et al. Potentially lethal ventricular arrhythmias and heart failure in arrhythmogenic right ventricular cardiomyopathy: What are the differences between men and women? JACC Clin Electrophysiol. 2016;2:546–555. doi: 10.1016/j.jacep.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Mazzanti A, Ng K, Faragli A, Maragna R, Chiodaroli E, Orphanou N, Monteforte N, Memmi M, Gambelli P, Novelli V, et al. Arrhythmogenic right ventricular cardiomyopathy: Clinical course and predictors of arrhythmic risk. J Am Coll Cardiol. 2016;68:2540–2550. doi: 10.1016/j.jacc.2016.09.951. [DOI] [PubMed] [Google Scholar]
- 9.Protonotarios A, Anastasakis A, Panagiotakos DB, Antoniades L, Syrris P, Vouliotis A, Stefanadis C, Tsatsopoulou A, McKenna WJ, Protonotarios N. Arrhythmic risk assessment in genotyped families with arrhythmogenic right ventricular cardiomyopathy. Europace. 2016;18:610–616. doi: 10.1093/europace/euv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan M. Correlating information contents of gene ontology terms to infer semantic similarity of gene products. Comput Math Methods Med 2014. 2014:891842. doi: 10.1155/2014/891842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du J, Yuan Z, Ma Z, Song J, Xie X, Chen Y. KEGG-PATH: Kyoto encyclopedia of genes and genomes-based pathway analysis using a path analysis model. Mol Biosyst. 2014;10:2441–2447. doi: 10.1039/C4MB00287C. [DOI] [PubMed] [Google Scholar]
- 12.Hasan MM, Kahveci T. Indexing a protein-protein interaction network expedites network alignment. BMC Bioinformaticss. 2015;16:326. doi: 10.1186/s12859-015-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaertner A, Schwientek P, Ellinghaus P, Summer H, Golz S, Kassner A, Schulz U, Gummert J, Milting H. Myocardial transcriptome analysis of human arrhythmogenic right ventricular cardiomyopathy. Physiol Genomics. 2012;44:99–109. doi: 10.1152/physiolgenomics.00094.2011. [DOI] [PubMed] [Google Scholar]
- 14.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, et al. NCBI GEO: Archive for functional genomics data sets-update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J. ClueGO: A cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformaticss. 2009;25:1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformaticss. 2003;4:2. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakeel M, Irfan M, Khan IA. Rare genetic mutations in Pakistani patients with dilated cardiomyopathy. Gene. 2018;673:134–139. doi: 10.1016/j.gene.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Man J, Barnett P, Christoffels VM. Structure and function of the Nppa-Nppb cluster locus during heart development and disease. Cell Mol Life Sci. 2018;75:1435–1444. doi: 10.1007/s00018-017-2737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidecker B, Lamirault G, Kasper EK, Wittstein IS, Champion HC, Breton E, Russell SD, Hall J, Kittleson MM, Baughman KL, Hare JM. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. Eur Heart J. 2010;31:1188–1196. doi: 10.1093/eurheartj/ehp549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinale FG, Janicki JS, Zile MR. Membrane-associated matrix proteolysis and heart failure. Circ Res. 2013;112:195–208. doi: 10.1161/CIRCRESAHA.112.266882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi RB, Zhang JY, Lu DX, Wang HD, Wang HH, Li CJ. Glycine receptors contribute to cytoprotection of glycine in myocardial cells. Chin Med J (Engl) 2007;120:915–921. doi: 10.1097/00029330-200705020-00012. [DOI] [PubMed] [Google Scholar]
- 25.Wagner S, Maier LS, Bers DM. Role of sodium and calcium dysregulation in tachyarrhythmias in sudden cardiac death. Circ Res. 2015;116:1956–1970. doi: 10.1161/CIRCRESAHA.116.304678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci USA. 2002;99:6210–6215. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Tang H, Wei EQ, Wang Z, Yang J, Yang R, Wang S, Zhang Y, Pitt GS, Zhang H, Wang C. Conditional knockout of Fgf13 in murine hearts increases arrhythmia susceptibility and reveals novel ion channel modulatory roles. J Mol Cell Cardiol. 2017;104:63–74. doi: 10.1016/j.yjmcc.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44((D1)):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishida K, Yamaguchi O, Otsu K. Degradation systems in heart failure. J Mol Cell Cardiol. 2015;84:212–222. doi: 10.1016/j.yjmcc.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Hidron A, Vogenthaler N, Santos-Preciado JI, Rodriguez-Morales AJ, Franco-Paredes C, Rassi A., Jr Cardiac involvement with parasitic infections. Clin Microbiol Rev. 2010;23:324–349. doi: 10.1128/CMR.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12:1764–1775. doi: 10.1111/jth.12730. [DOI] [PubMed] [Google Scholar]
- 32.Barbalic M, Schwartz GL, Chapman AB, Turner ST, Boerwinkle E. Kininogen gene (KNG) variation has a consistent effect on aldosterone response to antihypertensive drug therapy: The GERA study. Physiol Genomics. 2009;39:56–60. doi: 10.1152/physiolgenomics.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Sanchez CE. Non renal effects of aldosterone. Steroids. 2014;91:1–2. doi: 10.1016/j.steroids.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Qin CX, May LT, Li R, Cao N, Rosli S, Deo M, Alexander AE, Horlock D, Bourke JE, Yang YH, et al. Small-molecule-biased formyl peptide receptor agonist compound 17b protects against myocardial ischaemia-reperfusion injury in mice. Nat Commun. 2017;8:14232. doi: 10.1038/ncomms14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bojnik E, Kleczkowska P, Marron Fernandez de Velasco E, Corbani M, Babos F, Lipkowski AW, Magyar A, Benyhe S. Bioactivity studies on atypical natural opioid hexapeptides processed from proenkephalin (PENK) precursor polypeptides. Comp Biochem Physiol B Biochem Mol Biol. 2014;174:29–35. doi: 10.1016/j.cbpb.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Ng LL, Sandhu JK, Narayan H, Quinn PA, Squire IB, Davies JE, Bergmann A, Maisel A, Jones DJ. Proenkephalin and prognosis after acute myocardial infarction. J Am Coll Cardiol. 2014;63:280–289. doi: 10.1016/j.jacc.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Treskatsch S, Feldheiser A, Shaqura M, Dehe L, Habazettl H, Röpke TK, Shakibaei M, Schäfer M, Spies CD, Mousa SA. Cellular localization and adaptive changes of the cardiac delta opioid receptor system in an experimental model of heart failure in rats. Heart Vessels. 2016;31:241–250. doi: 10.1007/s00380-014-0620-6. [DOI] [PubMed] [Google Scholar]
- 38.Siong Chan DC, Cao TH, Ng LL. Proenkephalin in heart failure. Heart Fail Clin. 2018;14:1–11. doi: 10.1016/j.hfc.2017.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the Gene Expression Omnibus repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE29819).