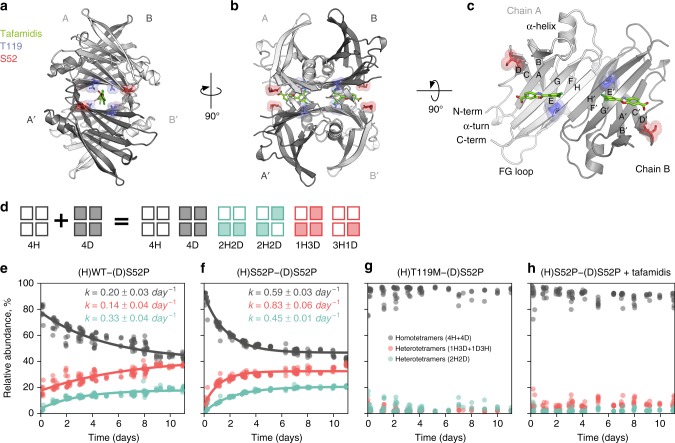
Fig. 1.
Structure of human TTR and experimental studies of TTR stability. a–c Crystallographic structure of WT-TTR (PDB-ID 4PVM). Tafamidis is shown in green (by superimposition of PDB-ID 6FFT), the S52 amino acid in red, and T119 in blue. d Scheme of the native MS experiment: 4H (hydrogenated) and 4D (deuterated) tetramers are mixed in equal parts; following dissociation, four hybrid tetrameric species may form by the assembly of monomeric and dimeric species. e–h MS result for the subunit exchange between deuterated S52P and hydrogenated WT, S52P, T119M, and S52P bound to tafamidis, respectively. Changes in relative abundance of homo- and hetero-tetrameric species over the course of 11 days are shown, along with an estimate of the of their association/dissociation rates (uncertainties are one standard deviation of the posterior densities)