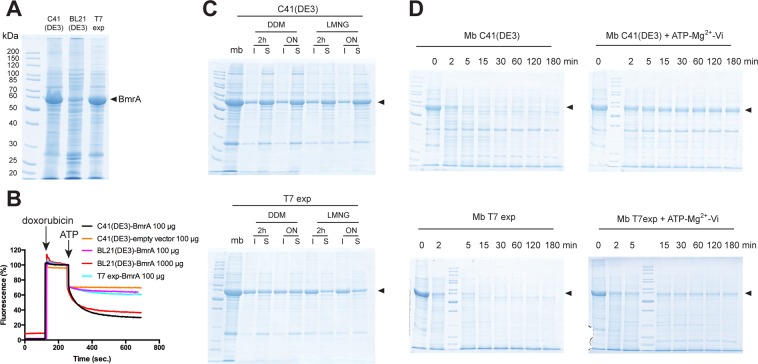
Figure 3.
Overexpression of BmrA in the membrane of various E. coli strains and functionality of the transporter. The expression of the transporter was induced by IPTG in exponential phase for 4 h. (A) Membrane protein expressions were visualized by 12% SDS-PAGE stained with Coomassie blue. Twenty µg of proteins were loaded in each lane. (B) Doxorubicin transport assays with inverted membrane vesicles prepared from the various strains. For the assays, 100 µg of total proteins were used for C41(DE3) and T7 express membranes, while 100 or 1000 µg were used for BL21(DE3) membranes. A representative experiment of 2–3 replicates is shown. (C) Detergent solubilization of membranes containing BmrA overexpressed from various strains. DDM or LMNG detergents were employed to extract BmrA from the membranes (10 µg of total proteins), either for 2 h or for overnight incubation. After ultracentrifugation, the soluble (S) and insoluble (I) fractions were submitted to 12% SDS-PAGE. Twenty µg of proteins were loaded in the membrane lane (mb). (D) Limited proteolysis of BmrA by trypsin. Membranes (Mb) from C41(DE3) or T7 express strains containing overexpressed BmrA were submitted to trypsin digestion for the indicated times, in the absence of ligand or after an incubation with ATP, Mg2+ and orthovanadate.