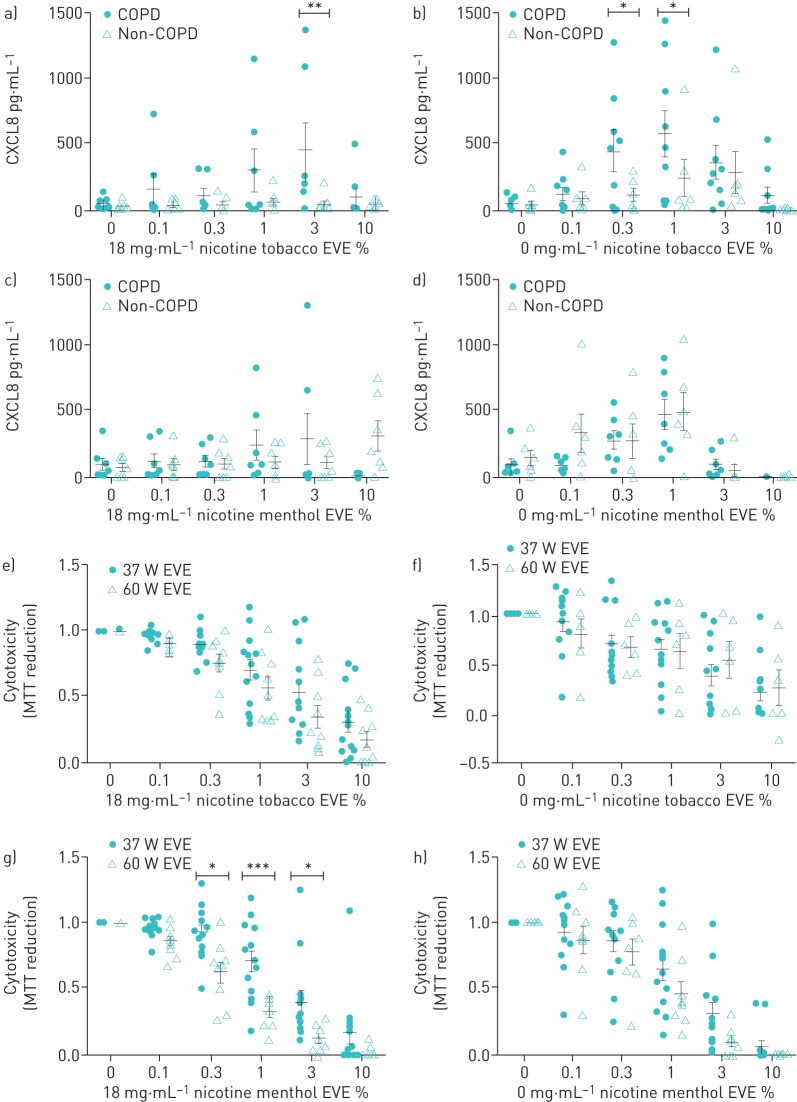
FIGURE 1.
Primary human airway smooth muscle cells were stimulated with different concentrations of a, e) 18 mg·mL−1 nicotine tobacco-flavoured e-cigarette vapour extract (EVE), b, f) 0 mg·mL−1 nicotine tobacco-flavoured EVE, c, g) 18 mg·mL−1 nicotine menthol-flavoured EVE and d, h) 0 mg·mL−1 nicotine menthol-flavoured EVE for 24 h. The control well contained 0.1% FBS DMEM. a–d) CXCL8 release production was measured in supernatant using ELISA. Error bars represent ±sem. Two-way ANOVA with Tukey's post-test was used for statistical analysis, significance is represented as *: p≤0.05; **: p≤0.01. e–h) Cytotoxicity was determined post-stimulation using an MTT assay. Data are normalised to unstimulated cells and error bars represent ±sem. Two-way ANOVA with Tukey's post-test was used for statistical analysis, significance is represented as *: p≤0.05; ***: p≤0.001. COPD: chronic obstructive pulmonary disease; MTT: 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.