Table 2. Group I click products of azidophenylsulfoxides 10 and azidophenyl sulfones 13 with selected arylacetylenes 16–24.
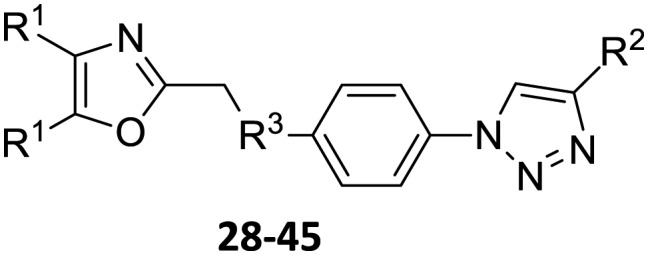
| |
| Compound | Yield a (%) |
| 28, R1 = Ph, R2 = 3-pyridyl, R3 = SO | 46 |
| 29, R1 = Ph, R2 = 4-fluorophenyl, R3 = SO | 86 |
| 30, R1 = Ph, R2 = 3-fluorophenyl, R3 = SO | 97 |
| 31, R1 = Ph, R2 = 2-methoxyphenyl, R3 = SO | 95 |
| 32, R1 = Ph, R2 = 2-(trifluoromethyl)phenyl, R3 = SO | 60 |
| 33, R1 = Ph, R2 = 6-methoxynaphthalene-2-yl, R3 = SO | 58 |
| 34, R1 = Ph, R2 = 4-(n-pentyl)phenyl, R3 = SO | 39 |
| 35, R1 = Ph, R2 = 4-(phenoxy)phenyl, R3 = SO | 79 |
| 36, R1 = Ph, R2 = 3,5-di-(trifluoromethyl)phenyl, R3 = SO | 89 |
| 37, R1 = Ph, R2 = 3-pyridyl, R3 = SO2 | 73 |
| 38, R1 = Ph, R2 = 4-fluorophenyl, R3 = SO2 | 63 |
| 39, R1 = Ph, R2 = 3-fluorophenyl, R3 = SO2 | 69 |
| 40, R1 = Ph, R2 = 2-methoxyphenyl, R3 = SO2 | 98 |
| 41, R1 = Ph, R2 = 2-(trifluoromethyl)phenyl, R3 = SO2 | 54 |
| 42, R1 = Ph, R2 = 6-methoxynaphthalene-2-yl, R3 = SO2 | 97 |
| 43, R1 = Ph, R2 = 4-(n-pentyl)phenyl, R3 = SO2 | 77 |
| 44, R1 = Ph, R2 = 4-(phenoxy)phenyl, R3 = SO2 | 97 |
| 45, R1 = Ph, R2 = 3,5-di-(trifluoromethyl)phenyl, R3 = SO2 | 63 |
aYields are for isolated pure compounds.
