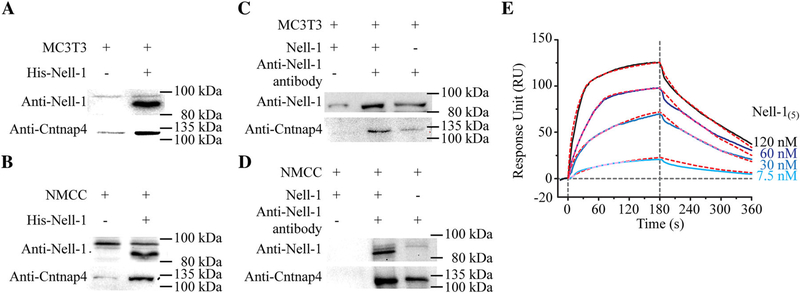
Fig. 4.
Physical interaction between Nell-1 and Cntnap4. Pull-down assays were performed with MC3T3-E1 pre-osteoblasts (A) and NMCC (B). Increased Cntnap4 was detected when beads were coated with His-tagged Nell-1. Co-immunoprecipitation assay with MC3T3-E1 pre-osteoblasts (C) and NMCC (D) demonstrated an increase in Cntnap4 when cells were incubated with Nell-1. To confirm specificity, no Cntnap4 was detected when the agarose beads were not coated with anti-Nell-1 antibody. (E) SPR assay was performed to assess the binding affinity and dynamic relationship between pentameric Nell-1 (Nell-1(5)) and the immobilized Cntnap4extra, which demonstrated classical ligand-receptor binding. Red dashed lines present the kinetic projection performed by Scrubber 2.0 (BioLogic Software Pty Ltd., Campbell, Australia).