Abstract
Background/Objective: Poor dietary quality, measured by the Healthy Eating Index 2010 (HEI-2010), is associated with risk of gestational diabetes mellitus (GDM) and type 2 diabetes. The aim was to investigate the association between dietary quality and glycemic control in women with GDM.
Materials and Methods: The study included 1220 women with GDM. Dietary quality was calculated by HEI-2010 score from a Food Frequency Questionnaire administered shortly after GDM diagnosis; higher scores indicate higher dietary quality. Subsequent glycemic control was defined as ≥80% of all capillary glucose measurements meeting recommended clinical targets below 95 mg/dL for fasting, and below 140 mg/dL 1-hour glucose after meals.
Results: As compared with Quartile 1 of HEI-2010 score, Quartiles 2, 3, and 4 showed increased adjusted odds of overall optimal glycemic control (odds ratio [95% confidence interval] 1.90 [1.34–2.70], 1.77 [1.25–2.52], and 1.55 [1.09–2.20], respectively). Increased odds of glycemic control were observed in Quartiles 2, 3, and 4 as compared with Quartile 1 of HEI-2010 score for 1-hour postbreakfast and 1-hour postdinner. Mean capillary glucose was lower in Quartiles 2, 3, and 4 of HEI-2010 score when compared with Quartile 1 for 1-hour postdinner (p = 0.03).
Conclusions: Clinicians should be aware that even a small improvement in diet quality may be beneficial for the achievement of improved glycemic control in women with GDM. Trial registration: Clinical Trials.gov number, NCT01344278.
Keywords: diet, gestational diabetes, glycemic control, nutrition, pregnancy
Introduction
Gestational diabetes mellitus (GDM) is a metabolic disorder of pregnancy that complicates, on average, 8% of pregnancies in the United States each year.1 GDM increases the rate of perinatal complications, such as macrosomia, shoulder dystocia, Cesarean section, and hypertension in pregnancy, and improving glycemic control during a pregnancy complicated by GDM can mitigate this risk.2
First-line treatment for GDM is focused on dietary therapies and lifestyle interventions to achieve glycemic control.3 Currently, medical nutrition therapy is centered around strict carbohydrate control to both improve glycemic control and lessen perinatal complications.4–6 However, evidence is conflicting on whether a low carbohydrate diet per se is the optimal dietary strategy for achieving glycemic control during a pregnancy complicated by GDM.7
Dietary quality is a gauge of overall dietary intake, rather than a focus on isolated nutrients.8 The Healthy Eating Index 2010 (HEI-2010)9,10 is a measure of dietary quality, reflective of the current Dietary Guidelines for Americans recommending nutrient-dense foods and beverages. Poor dietary quality has been associated with increased risk of GDM11 and type 2 diabetes risk after GDM.12 To our knowledge, the association of glycemic control during GDM with more global dietary quality measures has not previously been assessed. The aim of this investigation is to determine the association between dietary quality and glycemic control in women with GDM.
Materials and Methods
Source population
Our research question was examined in the Gestational Diabetes Effects on Moms (GEM) Study. The participants were recruited at Kaiser Permanente Northern California (KPNC), a large integrated health care delivery system consisting of 44 medical centers and 13 delivery hospitals with ∼33,000 deliveries per year. U.S. census data analysis shows that the race/ethnicity and education of KPNC members is representative of the geographic region served, and differ only slightly at the extremes of the income distribution.13,14
Detailed methods are described elsewhere; briefly, this was a cluster-randomized trial testing the comparative effectiveness of two Diabetes Prevention Programs (DPPs) implemented during the early postpartum period in women with GDM.15,16 GEM includes 2280 pregnant women, 18 years of age or older, with GDM (diagnosed by the Carpenter and Coustan criteria, as recommended by ACOG during the study period)17,18 identified during a 12-month period between March 27, 2011 and March 30, 2012 in the KPNC electronic health record (EHR). Women were sent a survey within 2 weeks after the diagnosis of GDM.
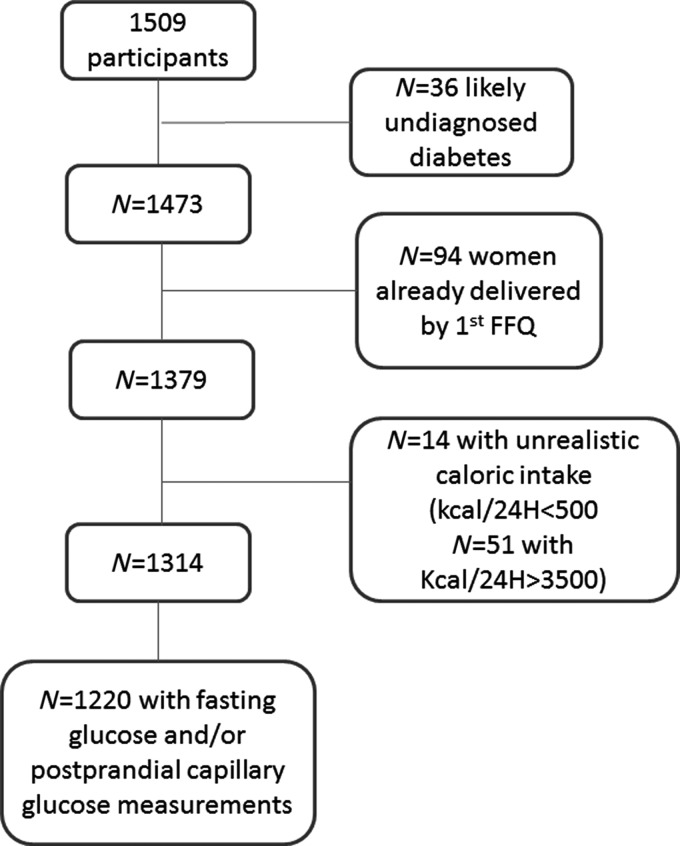
A total of 1783 women responded to the baseline survey (78.2%) in two phases. The first was conducted by a computer-assisted telephonic interview and the second was administered by mail, and included detailed diet and physical activity questionnaires. A total of 1509 women who returned a dietary assessment with <70 items blank were initially included in the analysis. Women were excluded if they reported unrealistic total energy intakes, such as <500 kcal/24-hour (n = 14), or >3500 kcal/24-hour (n = 51),19 or had already delivered before the dietary assessment (n = 94). A total of 1220 participants who completed the dietary measures and had at least one measure of fasting, 1-hour postbreakfast, 1-hour postlunch, and/or 1-hour postdinner self-assessed capillary glucose during the 6 weeks following the completion of the diet assessment were included in the analysis (Fig. 1).
FIG. 1.
This flowchart represents the study cohort assembly. FFQ, Food Frequency Questionnaire at baseline.
The Kaiser Foundation Research Institute Human Subjects Committee approved the trial and waived the requirement for informed consent for the intervention.
Exposure assessment
HEI-2010 dietary adherence scores were computed from the baseline Block Food Frequency Questionnaire (FFQ),20 which included culturally specific foods, administered at enrollment, and which reflected dietary intake over the 3 months before assessment. This FFQ has previously been validated in pregnant women, with Pearson correlation coefficients of r > 0.40 for antioxidants.21 This FFQ has also been validated and used with the Women, Infants, and Children nutritional supplementation program.22
Scoring criteria for the HEI-2010 are described in detail elsewhere.9 Briefly, the HEI-2010 is comprised of 12 dietary food group components with scores ranging from 0 to 20. Nine components are adequacy components, for which a higher score indicates higher dietary quality: Total fruit, whole fruit, vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, and a ratio of unsaturated to saturated fatty acids. Three components: refined grains, sodium, and empty calories (calories from solid fats, alcohol, and added sugars) are moderation components, for which consumption and score are ideally minimized. All the component scores were summed to obtain a total HEI-2010 score, which ranges from 0 to 100, with a score of 100 representing ideal dietary quality.
Outcomes
The primary outcome of interest is overall optimal glycemic control in the 6 weeks after completion of the dietary assessment. Overall optimal glycemic control was defined as ≥80% of all capillary glucose measurements meeting targets recommended in this clinical setting below 95 mg/dL for fasting, and below 140 mg/dL 1-hour glucose after meals (i.e., breakfast, lunch, and dinner). Optimal glycemic control for each measurement (fasting, 1 hour after breakfast, lunch, and dinner) was defined as ≥80% of all capillary glucose measurements for a given time point within the defined targets.
Data on capillary glucose values were obtained from self-monitoring data on fasting and 1-hour postprandial measurements maintained in a clinical database at the KPNC Regional Perinatal Service Center,23 which provides telephone-based case management to women with GDM in this health system. In this setting, all women with GDM are provided a standard glucometer and are recommended to self-monitor their glucose in the morning, while fasting, and 1 hour after breakfast, lunch, and dinner. Glucometers were calibrated when given to patients.
All study participants took part in the case management program, which instructs women to self-monitor and record glucose measurements in tracking booklets provided by the Center. Glucose measurements were then reported to the Center staff during weekly telephone counseling calls and data recorded in the Center's Patient-Reported Capillary Glucose (PRCG) clinical database. Fasting, 1-hour postbreakfast, 1-hour postlunch, and 1-hour postdinner glucose values reported in the 6-week period following completion of the FFQ were individually averaged and included in our analysis.
Covariates
Weight and height were measured in conjunction with pregravid and pregnancy routine care. Pregravid body mass index (BMI) was calculated from measured weight reported in the EHR (86%) or from self-reported pregravid weight at the time of the first prenatal clinic visit before 10 weeks of gestation (10.5%), or self-reported at the baseline survey (3.5%). Age, self-reported race ethnicity, and physical activity were obtained from the baseline GEM questionnaire. Physical activity was assessed using the Pregnancy and Physical Activity Questionnaire and is measured as metabolic equivalent (MET)-minutes exercise/week.24,25
Statistical analyses
Participants were divided into quartiles on the basis of HEI-2010 adherence scores. Differences between dietary pattern groups in baseline characteristics were assessed using a chi-square test (categorical variables) and univariate linear regression (continuous variables). Multivariable logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for associations between quartiles of dietary pattern adherence score and glycemic control. Multivariate models were adjusted for maternal age (years), race/ethnicity, physical activity (MET-minutes/week), and prepregnancy BMI (kg/m2) modeled as continuous outcomes and randomization condition. HEI-2010 score was normally distributed in each study arm and in the full participant group. Multiple linear regression models were used to test for differences in mean glucose values with increasing HEI score quartiles; fasting glucose, and glucose measured 1-hour postbreakfast, lunch and dinner were examined separately. Least square mean glucose levels for each HEI score quartile and between-quartile mean differences were calculated (lowest HEI-2010 score quartile level serving as the reference), along with their 95% CIs. The analysis was completed with the use of STATA version 11.2, 2012.
Results
A total of 1220 women with GDM who were patients at Kaiser Permanente Medical Center, who completed the dietary measures and had at least one measure of glycemic control during the 6 weeks following the completion of the Block FFQ were included. The mean age of participants was 32 years, and ∼40% of all participants were of Asian ethnicity. As compared with women in the lowest HEI-2010 score quartile, women in the highest quartile of HEI-2010 score were more likely to be older and of Asian or Hispanic ethnicity. Overall dietary quality as measured by the HEI-2010 had a mean score of 44 in Quartile 1 and 62 in Quartile 4 (Table 1). There was no difference in baseline HEI-2010 scores by randomization condition (54.3 vs. 53.7; p = 0.15).
Table 1.
Characteristics of Study Participants: The Gestational Diabetes Effects on Moms Study, Kaiser Permanente Northern California, 2011–2012
| HEI-2010 score quartile | p | |||||
|---|---|---|---|---|---|---|
| Quartile 1 (N = 305) | Quartile 2 (N = 305) | Quartile 3 (N = 305) | Quartile 4 (N = 305) | ANOVA | Chi-square | |
| Age, years | 32.50 (5.14) | 31.73 (5.04) | 32.53 (4.65) | 33.03 (4.52) | 0.03 | |
| Prepregnancy BMI, kg/m2 | 30.11 (7.06) | 29.46 (6.99) | 28.04 (6.65) | 27.13 (6.03) | 0.08 | |
| Race ethnicity, N (%) | 0.004 | |||||
| Non-Hispanic White | 72 (24) | 76 (25) | 88 (29) | 73 (24) | ||
| Hispanic | 48 (16) | 58 (19) | 58 (19) | 63 (21) | ||
| Asian | 135 (44) | 115 (38) | 111 (36) | 147 (48) | ||
| Black/African American | 17 (6) | 9 (3) | 9 (3) | 3 (1) | ||
| Other | 8 (2) | 7 (2) | 3 (1) | 5 (2) | ||
| Multiracial/multiethnic | 25 (8) | 40 (13) | 36 (12) | 14 (4) | ||
| Daily energy intake (kcal/24-hour) | 1771 (698) | 1612 (620) | 1692 (572) | 1702 (549) | 0.02 | |
| Physical activity (MET-minutes/week) | 1752 (1512) | 1727 (1423) | 1895 (1332) | 1796 (1315) | 0.07 | |
Data are mean (standard deviation) unless otherwise indicated.
ANOVA, analysis of variance; BMI, body mass index; MET, metabolic equivalent; HEI-2010, Healthy Eating Index 2010.
The primary outcome was the presence of optimal glycemic control. As shown in Table 2, in comparison with Quartile 1 of HEI-2010 score, there were increased odds of overall optimal glycemic control in Quartiles 2, 3, and 4 of HEI-2010 score (OR [95% CI] 1.90 [1.34–2.70]), (1.77 [1.25–2.52]), and (1.55 [1.09–2.20]), respectively.
Table 2.
Adjusted Odds of Achieving Optimal Glycemic Control by Quartile of Healthy Eating Index 2010 Score: The Gestational Diabetes Effects on Moms Study, Kaiser Permanente Northern California, 2011–2012
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Wald test | |||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) | OR | Prevalence (%) | OR (95% CI) | Prevalence (%) | OR (95% CI) | Prevalence (%) | OR (95% CI) | p | |
| Overall glycemic controla | 36 | REFb | 52 | 1.90 (1.34–2.70) | 51 | 1.77 (1.25–2.52) | 50 | 1.55 (1.09–2.20) | 0.01 |
| Met goal fasting glucose <95 mg/dL | 74 | REF | 80 | 1.52 (1.00–2.31) | 78 | 1.20 (0.79–1.81) | 79 | 1.08 (0.72–1.64) | 0.23 |
| Met goal 1-hour postbreakfast glucose <140 mg/dLc | 74 | REF | 85 | 2.12 (1.38–3.27) | 84 | 2.04 (1.32–3.17) | 85 | 2.06 (1.33–3.19) | <0.001 |
| Met goal 1-hour postlunch glucose <140 mg/dLc | 66 | REF | 79 | 1.72 (1.17–2.53) | 79 | 1.73 (1.17–2.56) | 74 | 1.41 (0.97–2.06) | 0.02 |
| Met goal 1-hour postdinner glucose <140 mg/dLc | 63 | REF | 74 | 1.62 (1.11–2.36) | 75 | 1.69 (1.15–2.50) | 74 | 1.49 (1.02–2.18) | 0.03 |
Adjusted for maternal age (years), race/ethnicity, physical activity (MET-minutes/week), prepregnancy BMI (kg/m2), and randomization condition.
Overall Optimal Glycemic Control: ≥80% of all fasting (<95 mg/dL) and 1-hour postprandial glucose measurements (<140 mg/dL) within designated range.
REF: reference category.
CI, confidence interval; OR, odds ratio.
Defined as ≥80% of all capillary glucose measurements for a given time point within the defined targets.
When examining individual measurements of glycemic control, increased odds of glycemic control were observed in Quartiles 2, 3, and 4 as compared with Quartile 1 of HEI-2010 score. Odds of glycemic control in Quartile 4 as compared with Quartile 1 postbreakfast (2.06 [1.33–3.19]) and dinner (1.49 [1.02–2.18]) were significantly higher. There was no association between HEI-2010 score quartile and odds of glycemic control for fasting glucose measurements.
Adjusted group mean capillary glucose values by quartile of HEI-2010 score, as well as the mean differences between of HEI-2010 score quartiles, are presented in Table 3. Mean capillary glucose was lower in Quartiles 2, 3, and 4 of HEI-2010 score when compared with Quartile 1 for 1-hour postdinner values (p = 0.002). No significant associations between quartiles of HEI-2010 score and mean fasting capillary glucose were found.
Table 3.
Adjusted Mean and Adjusted Difference in Capillary Glucose Levels by Quartile of Healthy Eating Index 2010 Score: The Gestational Diabetes Effects on Moms Study, Kaiser Permanente Northern California, 2011–2012
| HEI-2010 | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Wald test |
|---|---|---|---|---|---|
| Fasting glucose, mg/dL | |||||
| Meana capillary glucose (95% CI) | 85.80 (85.52 to 86.06) | 84.67 (84.42 to 84.92) | 83.91 (83.67 to 84.14) | 84.35 (84.14 to 84.56) | |
| Meana difference in capillary glucose (95% CI) | — | −0.91 (−2.05 to 0.24) | −1.28 (−2.44 to −0.12) | −0.60 (−1.74 to 0.56) | 0.17 |
| One-hour postbreakfast glucose, mg/dL | |||||
| Meana capillary glucose (95% CI) | 120.45 (120.26 to 120.64) | 118.05 (117.84 to 118.26) | 117.79 (117.61 to 117.98) | 118.53 (118.34 to 118.71) | |
| Meana difference in capillary glucose (95% CI) | — | −2.20 (−3.95 to −0.45) | −2.04 (−3.81 to −0.26) | −1.51 (−3.27 to 0.26) | 0.06 |
| One-hour postlunch glucose, mg/dL | |||||
| Meana capillary glucose (95% CI) | 122.48 (122.25 to 122.71) | 121.03 (120.80 to 121.27) | 120.32 (120.11 to 120.54) | 120.60 (120.38 to 120.82) | |
| Meana difference in capillary glucose (95% CI) | — | −1.17 (−2.77 to 0.43) | −1.37 (−2.99 to 0.25) | −1.33 (−2.94 to 0.28) | 0.30 |
| One-hour postdinner glucose, mg/dL | |||||
| Meana capillary glucose (95% CI) | 124.42 (124.11 to 124.73) | 121.36 (121.08 to 121.64) | 120.71 (120.46 to 120.96) | 121.22 (120.99 to 121.46) | |
| Meana difference in capillary glucose (95% CI) | — | −2.68 (−4.29 to −1.07) | −2.77 (−4.40 to −1.13) | −2.26 (−3.88 to −0.64) | 0.002 |
Adjusted for study arm, maternal age (years) at OGTT, race/ethnicity, physical activity (MET-minutes/week), prepregnancy BMI (kg/m2). OGTT, oral glucose tolerance test.
In further analyses of HEI-2010 score components, there was increased odds of glycemic control with total vegetable intake and green and beans intake (p < 0.05) (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/jwh). There was no association between fruit intake and glycemic control. In line with the overall analyses, no significant associations between quartiles of HEI-2010 score and mean fasting capillary glucose were found for HEI-2010 components.
There were no significant cross-sectional associations between HEI-2010 score and oral glucose tolerance test (OGTT) result (data not shown).
Discussion
Higher dietary quality was associated with improved overall glycemic control and postprandial glycemic control among women with GDM. These findings are robust since the association between HEI-2010 score and glycemic control showed improved odds of achieving the target capillary glucose measurements above the lowest quartile of HEI-2010 score. Higher HEI-2010 score was associated with lower mean postprandial capillary glucose measurements after dinner. No association between HEI-2010 and fasting capillary glycemic control was found. Overall, dietary quality at baseline was low among all participants. In HEI-2010 component analyses, there was an association between higher total vegetable intake and increased odds of glycemic control. The observed associations were independent of maternal age, race/ethnicity, prepregnancy BMI, and physical activity. Our findings suggest that improving dietary quality above the lowest quartile of HEI-2010 score may have an effect in improving glycemic control.
Dietary quality represents patterns of intake, rather than narrowly focusing on specific micro or macronutrients. The HEI-2010 is a measure of dietary quality, and is based on the USDA 2010 guidelines for optimal dietary intake. A higher HEI-2010 score reflects higher dietary quality. The components of the score include adequacy components, for which a higher intake is the goal, and moderation components, for which intake should ideally be minimized.9 The mean baseline HEI-2010 score in our population, 54.0, is lower than the U.S. average; data from the 2009 to 2010 NHANES examination show an average HEI-2010 score of 59.5 among nonpregnant women.9 Our HEI-2010 scores, while lower than the national average, are similar to two recent investigations of diet quality in pregnancy, and therefore, are likely representative of truly poor overall diet quality in pregnant women.26,27
Prior investigations have examined the associations of dietary quality with the incidence of GDM and type 2 diabetes in large, longitudinal cohort studies11,12,28; however, to our knowledge, no previous studies have examined its association with glycemic control among individuals with diagnosed GDM. Vegetable and fruit intake in particular has been associated with decreased risk for GDM and type 2 diabetes.29–31 In this analysis, both total vegetable intake and dark green vegetables, beans, and peas intake was associated with better glycemic control, suggesting that overall diet quality may be driven by consumption of vegetables. There was no association found for intake of fruit and glycemic control; this may reflect that fruit intake has neither a beneficial nor a deleterious influence on glycemic control. The Dietary Approaches to Stop Hypertension (DASH) diet, which emphasizes vegetable, fruit, whole grain, and low-fat dairy intake has also been linked with better pregnancy outcomes in women with GDM.32 Taken together, improving overall dietary quality can begin with the practical advice to increase vegetable intake for women with GDM.
High postprandial glucose is indicative of impaired glucose tolerance, which reflects a state of peripheral insulin resistance.33 The impact of diet on insulin resistance is well characterized, and forms the basis of a major component of the DPP goals focused on lowering dietary fat intake.34 Our investigation did not find an association between fasting capillary glucose and baseline dietary quality, signifying that dietary quality may not significantly impact hepatic glucose production in women with GDM.33 While elevated fasting glucose values in early pregnancy are strong predictors of the development of GDM and subsequent type 2 diabetes,35–37 postprandial glycemic control in pregnancy is independently associated with the development of macrosomia and is an important area of intervention.35,38 Additionally, women with prior GDM may have poorer dietary quality, identifying a population for whom a focus on improving diet quality may assist in risk reduction for GDM in subsequent pregnancies.28
Medical nutrition therapy is a hallmark of treatment for GDM to improve glycemic control and decrease perinatal complications. This dietary plan is designed to maintain glucose control through carbohydrate restriction. In recent years, however, observational and randomized controlled trials have shown conflicting evidence of the benefits of a carbohydrate-restricted diet for improved glycemic control and maternal and fetal outcomes.7,39–41 Furthermore, in a 2014 trial, advice to eat low glycemic index foods versus compliance with overall healthy eating did not result in improved pregnancy outcomes.42 Dietary quality scores may present an additional means of characterizing beneficial and adverse dietary components to aid in postprandial glycemic control, and have been shown to improve these measures in type 1 diabetes.43 HEI-2010 score during pregnancy has also been associated with a reduction in neonatal adiposity.26 We believe that assessment and improvement of dietary quality can be an additional means of population-based risk reduction.
This study has several limitations. Our comprehensive dietary assessment was completed soon after GDM diagnosis, and we are lacking subsequent dietary assessments. Our analysis established a temporal relationship between dietary quality and later glycemic control, suggesting that dietary habits and quality during the 3 months before the GDM diagnosis captured during second trimester of pregnancy may have persistent effects on GDM control. At baseline, the caloric intake of all participants was low, and may reflect recall bias in reporting of habitual intake, or a weakness of the FFQ itself. Although this measure is accounted for in our analysis, there is the possibility of residual confounding. Additionally, recall bias from self-report may dilute the association between HEI-2010 score and glycemic control above the level of the poorest quality diet. Lastly, an FFQ administered at baseline may not have captured changes in dietary quality during the observation period. The Pregnancy and Physical Activity Questionnaire is validated in gathering physical activity data in general pregnancy, not specifically among women with GDM.
The strengths of this study are the large population of women with GDM for whom data on diet and measurements of capillary glucose levels were available, the racial and ethnic diversity of the participants, and ability to control for several covariates, including physical activity.
Conclusion
Higher dietary quality at GDM diagnosis is associated with optimal overall glycemic control, especially postprandial glycemic control. Our findings suggest that improving dietary quality may be an additional means of achieving postprandial glycemic control in this population. Clinicians should be aware that even a small improvement in dietary quality may be beneficial for the achievement of optimal glycemic control in women with GDM, and thus it is worthwhile to recommend improvements in dietary quality, such as increasing the quantity and variety of vegetable and legume intake, replacing refined carbohydrates with whole grains, minimizing added sugar and transfat intake, and consuming a variety of protein sources, favoring plant and fish-based proteins.
Supplementary Material
Acknowledgments
The authors thank Dr. Charles Quesenberry for his guidance on the statistical analysis. This research was supported by grant R01 HS019367 from the Agency for Healthcare Research and Quality, by grant R18 DK067334 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ferrara also received support from grant P30 DK092924 from the National Institute of Diabetes and Digestive and Kidney Diseases; Dr. Samantha Ehrlich also received support from grant K01DK105106 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Susan Brown also received support from grant K01 DK099404 from the National Institute of Diabetes and Digestive and Kidney Diseases. The study sponsors were not involved in the study design; data collection, analysis, and interpretation; writing of the report; or the decision to submit the article for publication.
Author Contributions
The roles for each author are as follows. M.D.G., S.F.E., and A.F. conceived of the analytic design and M.D.G. and Y.Z. performed the analysis; M.M.H., S.F.E., S.D.B., and A.F. contributed to the interpretation of the results and reviewed and edited the article; M.D.G. wrote the article; and M.D.G., S.F.E., and A.F. conceived of the project idea. M.D.G. had primary responsibility for final content. All authors have read and approved the final article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ferrara A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care 2007;30(Suppl 2):141. [DOI] [PubMed] [Google Scholar]
- 2. Landon MB, Spong CY, Thom E, et al. . A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association. 12. Management of diabetes in pregnancy. Diabetes Care 2015;39(Suppl 1):S98. [DOI] [PubMed] [Google Scholar]
- 4. Metzger BE, Buchanan TA, Coustan DR, et al. . Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl 2):251. [DOI] [PubMed] [Google Scholar]
- 5. Reader DM. Medical nutrition therapy and lifestyle interventions. Diabetes Care 2007;30(Suppl 2):188. [DOI] [PubMed] [Google Scholar]
- 6. Duarte-Gardea MO, Gonzales-Pacheco DM, Reader DM, et al. . Academy of Nutrition and Dietetics gestational diabetes evidence-based nutrition practice guideline. J Acad Nutr Diet 2018;118:1719–1742 [DOI] [PubMed] [Google Scholar]
- 7. Moreno-Castilla C, Hernandez M, Bergua M, et al. . Low-carbohydrate diet for the treatment of gestational diabetes mellitus: A randomized controlled trial. Diabetes Care 2013;36:2233–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu FB. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9 [DOI] [PubMed] [Google Scholar]
- 9. Guenther PM, Casavale KO, Reedy J, et al. . Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 2013;113:569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guenther PM, Kirkpatrick SI, Reedy J, et al. . The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr 2014;144:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tobias DK, Zhang C, Chavarro J, et al. . Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr 2012;96:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med 2012;172:1566–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health 1992;82:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Go AS, Hylek EM, Phillips KA, et al. . Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA 2001;285:2370–2375 [DOI] [PubMed] [Google Scholar]
- 15. Ferrara A, Hedderson MM, Albright CL, et al. . A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: Design and rationale of the gestational diabetes' effects on moms (GEM) study. BMC Pregnancy Childbirth 2014;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrara A, Hedderson MM, Brown SD, et al. . The comparative effectiveness of diabetes prevention strategies to reduce postpartum weight retention in women with gestational diabetes mellitus: The gestational diabetes' effects on moms (GEM) cluster randomized controlled trial. Diabetes Care 2016;39:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Committee opinion No. 504: Screening and diagnosis of gestational diabetes mellitus. Obstet Gynecol 2011;118:751–753 [DOI] [PubMed] [Google Scholar]
- 18. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768–773 [DOI] [PubMed] [Google Scholar]
- 19. Chia AR, Tint MT, Han CY, et al. . Adherence to a Healthy Eating Index for pregnant women is associated with lower neonatal adiposity in a multiethnic Asian cohort: The Growing Up in Singapore Towards Healthy Outcomes (GUSTO) study. Am J Clin Nutr 2018;107:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–469 [DOI] [PubMed] [Google Scholar]
- 21. Brunst K, Kannan S, Ni Y, Gennings C, Ganguri H, Wright R. Validation of a Food Frequency Questionnaire for estimating micronutrient intakes in an urban US sample of multi-ethnic pregnant women. Matern Child Health J 2016;20:250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Summary of WIC nutrition risk criteria: A scientific assessment. Committee on Scientific Evaluation of WIC Nutrition Risk Criteria Food and Nutrition Board, Institute of Medicine, National Academy of Sciences. J Am Diet Assoc 1996;96:925–930 [PubMed] [Google Scholar]
- 23. Ferrara A, Hedderson MM, Ching J, Kim C, Peng T, Crites YM. Referral to telephonic nurse management improves outcomes in women with gestational diabetes. Am J Obstet Gynecol 2012;206:491..e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown WJ, Bauman AE. Comparison of estimates of population levels of physical activity using two measures. Aust N Z J Public Health 2000;24:520–525 [DOI] [PubMed] [Google Scholar]
- 25. Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc 2004;36:1750–1760 [DOI] [PubMed] [Google Scholar]
- 26. Shapiro AL, Kaar JL, Crume TL, et al. . Maternal diet quality in pregnancy and neonatal adiposity: The healthy start study. Int J Obes (Lond) 2016;40:1056–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shin D, Lee KW, Song WO. Pre-pregnancy weight status is associated with diet quality and nutritional biomarkers during pregnancy. Nutrients 2016;8:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao RS, Simas TA, Person SD, Goldberg RJ, Waring ME. Diet quality and history of gestational diabetes mellitus among childbearing women, United States, 2007–2010. Prev Chronic Dis 2015;12:E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010;341:c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cooper AJ, Forouhi NG, Ye Z, et al. . Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr 2012;66:1082–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: Review of epidemiologic evidence. Am J Clin Nutr 2011;94(6 Suppl):1979S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Asemi Z, Samimi M, Tabassi Z, Esmaillzadeh A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: A randomized controlled clinical trial. Eur J Clin Nutr 2014;68:490–495 [DOI] [PubMed] [Google Scholar]
- 33. Nathan DM, Davidson MB, DeFronzo RA, et al. . Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 34. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kramer CK, Swaminathan B, Hanley AJ, et al. . Each degree of glucose intolerance in pregnancy predicts distinct trajectories of β-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care 2014;37:3262–3269 [DOI] [PubMed] [Google Scholar]
- 36. Mills JL, Jovanovic L, Knopp R, et al. . Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: The diabetes in early pregnancy study. Metabolism 1998;47:1140–1144 [DOI] [PubMed] [Google Scholar]
- 37. Pertot T, Molyneaux L, Tan K, Ross GP, Yue DK, Wong J. Can common clinical parameters be used to identify patients who will need insulin treatment in gestational diabetes mellitus? Diabetes Care 2011;34:2214–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Combs CA, Gunderson E, Kitzmiller JL, Gavin LA, Main EK. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care 1992;15:1251–1257 [DOI] [PubMed] [Google Scholar]
- 39. Major CA, Henry MJ, De Veciana M, Morgan MA. The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes. Obstet Gynecol 1998;91:600–604 [DOI] [PubMed] [Google Scholar]
- 40. Hernandez TL, Mande A, Barbour LA. Nutrition therapy within and beyond gestational diabetes. Diabetes Res Clin Pract 2018. DOI: S0168-822730370-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hernandez TL, Van Pelt RE, Anderson MA, et al. . Women with gestational diabetes mellitus randomized to a higher-complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: A pilot study. Diabetes Care 2016;39:39–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moses RG, Casey SA, Quinn EG, et al. . Pregnancy and glycemic index outcomes study: Effects of low glycemic index compared with conventional dietary advice on selected pregnancy outcomes. Am J Clin Nutr 2014;99:517–523 [DOI] [PubMed] [Google Scholar]
- 43. Nansel TR, Lipsky LM, Liu A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am J Clin Nutr 2016;104:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.