Abstract
Depression and anxiety can be severely detrimental to the health of both the affected woman and her offspring. In a rodent model of postpartum depression and anxiety, chronic social stress exposure during lactation induces deficits in maternal care and increases anxiety. Here, we extend previous findings by expanding the behavioral analyses, assessing lactation, and examining several neural systems within amygdalar and hypothalamic regions involved in the control of the stress response and expression of maternal care that may be mediating the behavioral changes in stressed dams. Compared with control dams, those exposed to chronic social stress beginning on day 2 of lactation show impaired maternal care and lactation and increased maternal anxiety on day 9 of lactation. Saccharin-based anhedonia and maternal aggression were increased and lactation was also impaired on day 16 of lactation. These behavioral changes were correlated with a decrease in oxytocin mRNA expression in the medial amygdala, and increases in the expressions of corticotrophin-releasing hormone mRNA in the central nucleus of the amygdala, glucocorticoid receptor mRNA in the paraventricular nucleus, and orexin 2 receptor mRNA in the supraoptic nucleus of stressed compared with control dams. The increase in glucocorticoid receptor mRNA in the paraventricular nucleus was negatively correlated with methylation of a CpG site in the promoter region. In conclusion, the data support the hypothesis that social stress during lactation can have profound effects on maternal care, lactation, and anxiety, and that these behavioral effects are mediated by central changes in stress and maternally relevant neuropeptide systems.
Keywords: anxiety, gene expression, lactation, maternal care, postpartum depression, rat, social stress
Introduction
Maternal disorders such as postpartum depression and anxiety (PPD/A) affect women within 4 weeks of childbirth. Prevalence rates vary from 10 to 20% in most populations (O’Hara and McCabe, 2013; O’Hara and Wisner, 2014), but are higher than 30% in some populations, such as among women living in rural areas of developing countries (Villegas et al., 2011) and among low-income, ethnic minority women (Gress-Smith et al., 2012). These disorders can have robust adverse effects on the health and well-being of both the mother and the offspring. The growing literature has demonstrated associations between PPD/A and adverse child outcomes (Grace et al., 2003), with consequences not restricted to infancy, but also extending into adolescence and even adulthood, often mediated by decreases in reward-mediated maternal care in the depressed mothers (Goodman et al., 2011). Infants of depressed mothers show impaired maternal–child interactions and behavioral difficulties (Forman et al., 2007), cognitive delays (Sohr-Preston and Scaramella, 2006), a high level of stress reactivity (Feldman et al., 2009), and a higher risk of psychiatric disorders during adolescent years than those of nondepressed mothers (Pearson et al., 2013). There is an imminent need for preclinical studies investigating the mechanisms underlying PPD/A to develop improved preventative measures and treatments for these disorders. Improving mental health in mothers also represents a preventative measure to ensure the health of their offspring.
One key predictor of PPD/A is exposure to interpersonal stressors such as social conflict and lack of social support (O’Hara and Wisner, 2014). We have previously reported that social conflict can be mimicked in maternal rodents using a model of chronic social stress (CSS), and that it can reliably depress maternal care (Nephew and Bridges, 2011; Carini et al., 2013). In this paradigm, lactating rat dams are confronted with a male intruder placed in their home cage for 1 h daily between days 2 and 16 of lactation. The newborn pups remain in the cage while the resident dam aggressively defends her litter against the male intruder. Exposure to this ethologically and translationally relevant stressor depresses maternal care on day 9 of lactation. In the CSS model, maternal care is used as a primary measure of anhedonia because of its robust reward-mediated behavioral and neural aspects (Mattson et al., 2001; Ferris et al., 2005) and its critical relevance to the symptomology of PPD/A, as maternal care is often impaired in depressed mothers and mediates the adverse effects of depression and anxiety on offspring (O’Hara and McCabe, 2013; O’Hara and Wisner, 2014). The CSS model augments the related literature on chronic stress and exogenous corticosterone-based rodent models of PPD (Perani and Slattery, 2014) using an ethologically relevant stressor and maternal behavior and other home cage behaviors to assess depression and anxiety to enhance construct and face validity. Exposure of the pups to this combined social stress of impaired maternal care and social conflict is also a clinically relevant form of early-life stress, which can lead to the development of depressed maternal care and anxiety in humans (Essex et al., 2011). Compared with dams not exposed to CSS as pups, early-life social stress-exposed dams exhibit depressed maternal care, impaired lactation, and increased restlessness (Carini and Nephew, 2013; Murgatroyd and Nephew, 2013), and these reports support the hypothesis that CSS has maladaptive effects on both the mother and the offspring, similar to PPD/A in humans (O’Hara and McCabe, 2013; O’Hara and Wisner, 2014).
In this study, we expand on our prior knowledge of the behavioral effects of CSS and investigate long-term changes in several neural systems implicated in impaired social interactions, responses to chronic stress, and the expression of maternal care and maternal aggression. The neurohormones arginine vasopressin (AVP) and oxytocin (OXT) both have well-known links to the expression of maternal and related social behaviors such as maternal aggression and infant bonding, respectively (Young, 1999; Insel and Young, 2001; Donaldson and Young, 2008; Nephew, 2012). Corticotrophinreleasing hormone (CRH), the central effector of the hypothalamic–pituitary–adrenal (HPA) axis and the key mediator of fear and anxiety states, has been implicated in the adverse effects of psychological stress in both animals and humans (Lupien et al., 2009). Furthermore, the role of the glucocorticoid receptor (GR) in the transgenerational transmission of maternal care has been extensively investigated in rodents (Zhang et al., 2013). However, most of this work has focused on the effects of altered maternal care on offspring behavior and neuroendocrinology, not on the effects of stress on the mother. In addition, the orexin/hypocretin system, such as the expression of orexin and its receptors (Orx1r and Orx2r), has been implicated in the expression of both maternal care (D’Anna and Gammie, 2006) and depressive behavior and pathophysiology (Arendt et al., 2013; Nollet and Leman, 2013), but whether this system is involved in the decreased expression of maternal care observed in our rodent model of CSS is unknown. It is suggested that increased focus on the mother may reveal novel safe and effective preventative interventions aimed at ameliorating the adverse effects of PPD/A on offspring. It was hypothesized that these behaviorally relevant neural systems are altered in amygdalar and hypothalamic regions within the brains of dams that were exposed to CSS during lactation compared with control dams, and that the observed changes in these systems are correlated with related behavioral deficits.
Methods
Subjects
Sprague–Dawley rats in this study were maintained in accordance with the guidelines of the Committee for the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee. Food and water were provided freely, and the light cycle was 12 h light/12 h darkness, with lights on at 07.00 h. All rats were purchased as adults (Charles River, Wilmington, Massachusetts, USA), mated with breeder males, and housed in groups of three until the day before parturition. Litters were culled to five male and five female rats on the day of parturition. One randomly selected pup was taken from each litter and killed on days 2, 9, and 16 (alternating between sexes) for postmortem milk extraction at the end of the 120-min nursing period. For behavioral testing, litters included 10 pups on day 2, nine pups on day 9, and eight pups on day 16. Dams were euthanized on day 23 at weaning between 08.00 and 10.00 h, and trunk blood and brains were collected at this time. Brains were frozen at − 80°C and then micropunched to obtain samples of the paraventricular nucleus (PVN), the supraoptic nucleus (SON), the central amygdala (CeA), and the medial amygdala (MeA) for PCR analysis of the relative mRNA levels. The sample sizes for the dams were 11 control and 13 CSS samples.
Chronic social stress model
The CSS dams were subjected to a CSS protocol from days 2 to 16 of lactation, as reported previously (Nephew and Bridges, 2011; Carini et al., 2013). This procedure included placing a similarly sized (220–300 g) novel Sprague–Dawley male intruder into a lactating female’s home cage (10.5-inch W × 19-inch D × 8-inch H) for 1 h from days 2 to 16 of lactation. Control dams were not exposed to the CSS protocol; they were only tested for maternal care and maternal aggression on days 2, 9, and 16 of lactation. The pups were left in the cage during intruder presentation.
Maternal care test
Maternal care and maternal aggression were assessed in all dams between 09.00 and 12.00 h on days 2, 9, and 16 of lactation (early, mid, and late lactation) to assess the effects of early-life CSS at different time points during lactation (Carini et al., 2013; Murgatroyd and Nephew, 2013). After a 60-min pup removal, maternal care testing was performed, which included reintroduction of all pups into the home cage and video recording of the dam for 30 min These 30-min behavioral observations produce consistent and substantial behavioral data that are similar to the observations from undisturbed maternal care over 30 and 60 min (Byrnes et al., 2000; Johnson et al., 2011). Frequencies and durations of pup retrieval, pup grooming, nursing, nesting, self-grooming, and general locomotor activity were scored by an observer who was blind to the treatment, using ODLog behavioral analysis software (Macropod Inc., New York, NY, USA). Scoring of nursing behavior was started when the dam had been motionless over the litter for longer than 10 s and stopped whenever she moved off the pups. Nursing duration is the cumulative nursing behavior during the 30-min maternal care observation. The mean duration of nursing and self-grooming bouts was calculated for each animal by dividing duration by frequency. Total maternal care included the combined durations of pup grooming and nursing. Nesting was defined as manipulation of the nesting material with the mouth or paws. The combined durations of self-grooming, nesting, and locomotor activity during maternal care testing is referred to as maternal anxiety. Following the 30-min maternal care video recording, the pups were left undisturbed for another 90 min (without video recording) to provide an undisturbed 120-min nursing period to assess milk intake by weighing the litter. Milk intake was calculated by subtracting the litter weight at the start of maternal care observation from the litter weight 120 min later.
Maternal aggression test
Following maternal care testing and the nursing period, the pups were weighed, returned to the cage, and a similarly sized (225–300 g) novel virgin male Sprague–Dawley intruder was introduced for a 30-min maternal aggression test. Dams were never exposed to the same male twice through the rotating use of 20 intruder males. The latency to initiate aggression and the frequency and duration of attacking (boxing or tackling), biting, kicking, pinning to the bottom of the cage, selfgrooming, and locomotor activity were scored. Total aggression included the durations of attacking, biting, kicking, and pinning to the bottom of the cage.
Saccharin preference testing
Preweighed bottles of water and 0.02% saccharin were placed in the dams’ cages at 16.00 h on lactation days 2, 9, and 16 (alternating left or right sides of the cage). The bottles were removed and weighed the next morning at 08.00 h to determine the % of saccharin intake out of total fluid intake over the 16- h period.
Milk caloric density
One pup from each litter was killed by decapitation at the end of the nursing period on days 2, 9, and 16, and the contents of its stomach were emptied into a 5 ml Eppendorf tube through an incision at the anterior end of the stomach and frozen at − 80°C for bomb calorimetric analyses. Milk samples from days 6 and 16 from each animal were all combined within treatment to create two samples (one control, one CSS) large enough to be weighed, homogenized, freeze dried (Virtis Benchmark 1000 Lyophilizer; Virtis Co, Gardiner, New York), ground to a fine powder, and then analyzed in duplicate for gross energy using an Isoperibol Bomb Calorimeter (Parr model 1261; Parr Instrument Co., Moline, Illinois, USA). Benzoic acid standards (benzoic acid 1 g pellets; Parr Instrument Co.) were analyzed, and values were within 1% of the known heat of combustion for benzoic acid. The gross energy content of both samples was calculated by multiplying the average kilocalories per gram of dry solids by the total weight of dry solids.
RNA expression analyses
Total RNA and DNA were simultaneously extracted (Bettscheider et al., 2011), and reverse transcription reactions were performed on 100 ng RNA using the Tetro Reverse Transcriptase kit (Bioline) and oligo(dT)primer to analyze transcript levels. Quantitative PCR was performed in triplicate on StepOne Plus (Life Technologies, UK) using Sensi Fast SYBR green (Bioline, UK). Primer sequences were as follows: Avp1aR: F-CAGCAGCGTGAAGAGCATTT, R-CGCC GTGATTGTGATGGAAG; Oxt: F-TCTGACCTCCG CCTGCTACATC, R-AAGCAGCGCCTTTGCCGCC; OxtR: F-GTACTGGCCTTCATCGTGTGC, R-TGCA GCAGCTGTTGAGGCTG; Orx1r: F-AGGTGGATGGA AGCGTGAAG, R-AGAGATAATCGCGCCACAGG; Orx2r: F-CGCAACTGGTCATCTGCTTC, R-TTCGT GCTCGGATCTGCTTT; Prl: F-GTAGATGGA GCCAGGAGAGTTC, R-ACCAGAGTCACTGTCG GGATCT; Avp: F-CAGATGCTCGGCCCGAAG, R-TTCCAGAACTGCCCAAGAG; Crh: F-CAGAACAA CAGTGCGGGCTCA, R-AAGGCAGACAGGGCGA CAGAG; β-actin: F-TTGCTGACAGGATGCAGAA, R-ACCAATCCACACAGAGTACTT; Hprt: F-TGGTC AAGCAGTACAGCCCC, R-TACTGGCCACATCAA CAGGA; Gapdh: F-CATCACCATCTTCCAGGAGC, R-TAAGCAGTTGGTGGTGCAGG; Nr3c1: F-AGG GGAGGGGGAGCGTAATGG, R-CCTCTGCTGCTT GGAATCTGC; Nr3c2: F-TACGACAATTCCAAGCC CGACACC, R-TACCTTGGCCCACTTCACGACCTG; Mrfap: F-CATCTGGGACTGTGGATGGG, R-CCAACAAACTCATCAGGCAGG; Pgk1: F-GAAGGGAA GGGAAAAGATG, R-AAATCCACCAGCCTTCTG TG. Conditions were 94°C for 2 min, followed by 40 cycles of 94°C for 5 s, between 58 and 64°C for 5 s, and 72°C for 10 s. Levels were normalized against the combined housekeeping genes β-actin, hypoxanthine phosphoribosyltransferase (Hprt), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) for SON and PVN studies, and Morf4 family associated protein 1 (Mrfap) and phosphoglycerate kinase 1 (PGK1) for amygdalar regions.
DNA methylation
Genomic DNA was extracted as previously described (Bettscheider et al., 2011) and 200 μg DNA was bisulfitemodified using the EpiTect Bisulfite Kit (Qiagen). Amplification of a 137-bp region within the rat Nr3c1 promoter was performed with specific primers (F-ACT CCCCAACCATTCCAAACCTC; R-biotin-CCAACCTCCCTAACATATCCT), using the PyroMark PCR Kit (without Q solution); the conditions were: 40 cycles of 94° C for 30 s, 56°C for 30 s, and 72°C for 30 s. Single-stranded biotinylated product was purified by mixing 10 µl of the amplification mixture, 2 µl of streptavidin sepharose HP (Amersham Biosciences), and 40 µl of binding buffer. The sepharose beads containing the immobilized biotinylated product were purified, washed, and denatured in 0.2 mol/l NaOH and washed again using the Pyrosequencing Vacuum Prep Tool (Qiagen). The biotinylated DNA was resuspended in 12 µl of annealing buffer containing 0.3 µmol/l pyrosequencing primer ( ACTCCCCAACCATTCCAAACCTC). DNA methylation of three CpG residues within the Nr3c1 gene promoter (RGSC 5.0/rn5; chr18:32,351,454–32,351,474) was quantified by pyrosequencing using the PSQ 24MA system with the PyroGold SQA reagent kit (Qiagen). The percentage methylation for each of the CpG sites was calculated using Pyro Q-CpG software (Qiagen).
Endocrine assays
Plasma corticosterone and estradiol levels were measured using commercial radioimmunoassay kits (Siemens, New York, NY); prolactin levels were measured using the radioimmunoassay kit provided by Dr A.F. Parlow at the National Hormone and Peptide Program, Harbor-UCLA Medical Center; and oxytocin levels were measured using a commercial enzyme-linked immunosorbent assay kit (Enzo Life Sciences, Farmingdale, NY). All samples were run in duplicate. Assay sensitivities were as follows: corticosterone, 5.7 ng/ml; prolactin, 0.5 ng/ml; estradiol, 8 pg/ml; and oxytocin, 11.7 pg/ml. Intra-assay variability was 3–7%, and number of comparisons and the specific focus on significant differences in gene expression and behavior. All graphical results are presented as mean ± SEM, and the interassay variability was 5–10%.
Statistics
Dam and pup bodyweight and growth, maternal behaviors, milk intake, and saccharin preference were tested using individual analyses of variance on each day of lactation to independently test for differences on each day. Independent comparisons of data from day 2 were necessary to determine whether there were any differences between the control and CSS groups before the start of the CSS protocol. Relative mRNA expression levels were compared using individual t-tests for each brain region. Pearson correlations were used to test for significant gene–gene (six tests) and gene–behavior associations (eight tests: maternal care and maternal anxiety with the four significant differences in gene expression shown in Fig. 4). Corrections for multiple comparisons were not performed because of the low number of comparisons and the specific focus on significant differences in gene expression and behavior. All graphical results are presented as mean ± SEM, and the level of statistical significance was P less than 0.05.
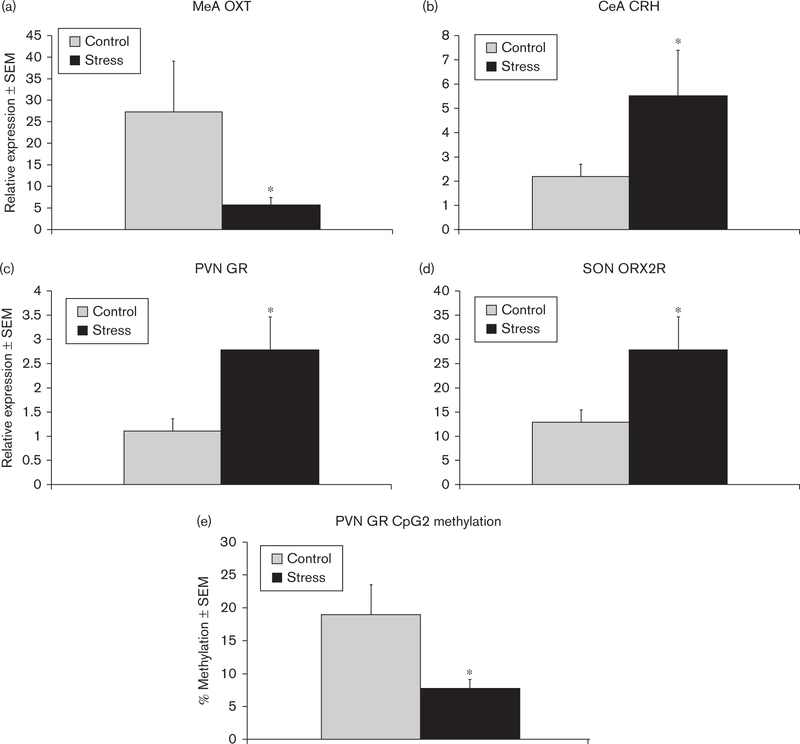
Fig. 4.
Mean ± SEM relative expression of (a) OXT mRNA in the MeA, (b) CRH mRNA in the CeA, (c) GR mRNA in the PVN, and (d) ORX2R mRNA in the SON of control and early-life CSS (stress) dams. (e) Mean percentage methylation of the GR CpG2 was decreased in the PVN of CSS dams. *Significant effect of CSS, P < 0.05. CeA, central amygdale; CRH, corticotrophin-releasing hormone; CSS, chronic social stress; GR, glucocorticoid receptor; MeA, medial amygdale; ORX, orexin; OXT, oxytocin; PVN, paraventricular nucleus; SON, supraoptic nucleus.
Results
Dam and pup growth
There were no significant differences in dam growth during lactation and in pup bodyweight at the start of the stress protocol on day 2 (P > 0.1). Although there was no significant change in bodyweight between days 2 and 16 (P > 0.1), the CSS pups grew less over days 9–16 of lactation (day 16 weight/day 9 weight: control = 198.8 ± 1.7%, CSS = 191.3 ± 1.7%, F1,23 = 6.98, P < 0.01).
Maternal care testing
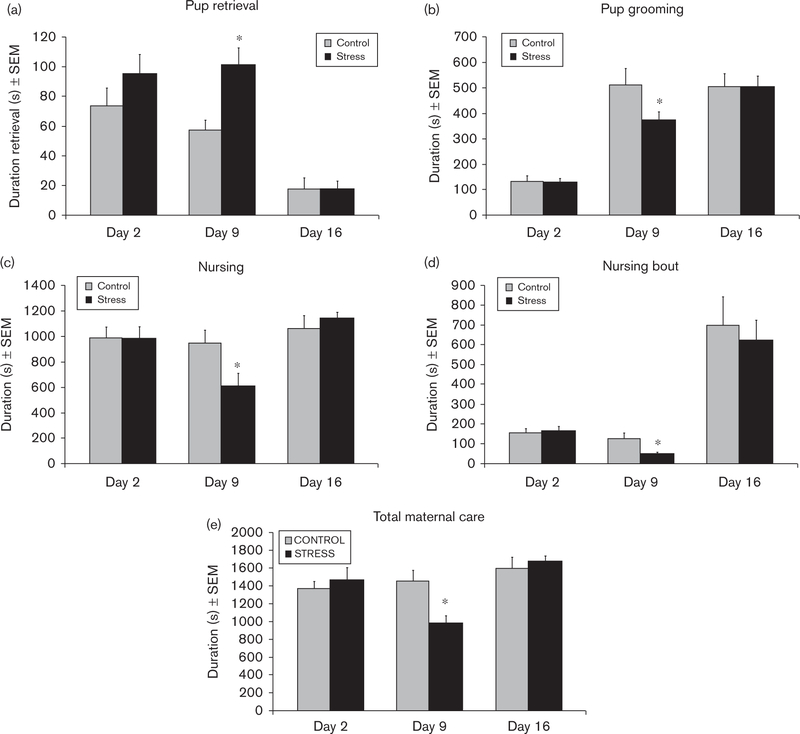
There were no significant differences in maternal care on day 2 of lactation before the start of the CSS protocol (all P’s > 0.2; Fig. 1). CSS dams spent more time retrieving the eight pups to the nest (F1,23 = 9.64, P < 0.01; Fig. 1a) and less time grooming (F1,23 = 4.4, P < 0.01; Fig. 1b) and nursing (F1,23 = 5.7, P < 0.01; Fig. 1c) during the 30-min maternal care test on day 9. The mean nursing bout duration was also attenuated in CSS dams on day 9 (F1,23 = 8.1, P < 0.01; Fig. 1d). The decreases in pup grooming and nursing led to an overall decrease in total maternal care (F1,23 = 11.5, P < 0.01; Fig.1e). There were no significant differences in maternal care on day 16 of lactation (all P’s > 0.3; Fig. 1). The increase in retrieval time indicates that it took longer for the CSS dams to gather their pups to the nest before grooming and nursing. This sometimes involved relocation of the nest, which is rarely observed in control dams.
Fig. 1.
Mean duration ± SEM of pup retrieval, pup grooming, nursing, nursing bout, and total maternal care during 30-min maternal care observations on lactation days 2, 9, and 16. *Significant effect of CSS on that lactation day, P < 0.05. CSS, chronic social stress.
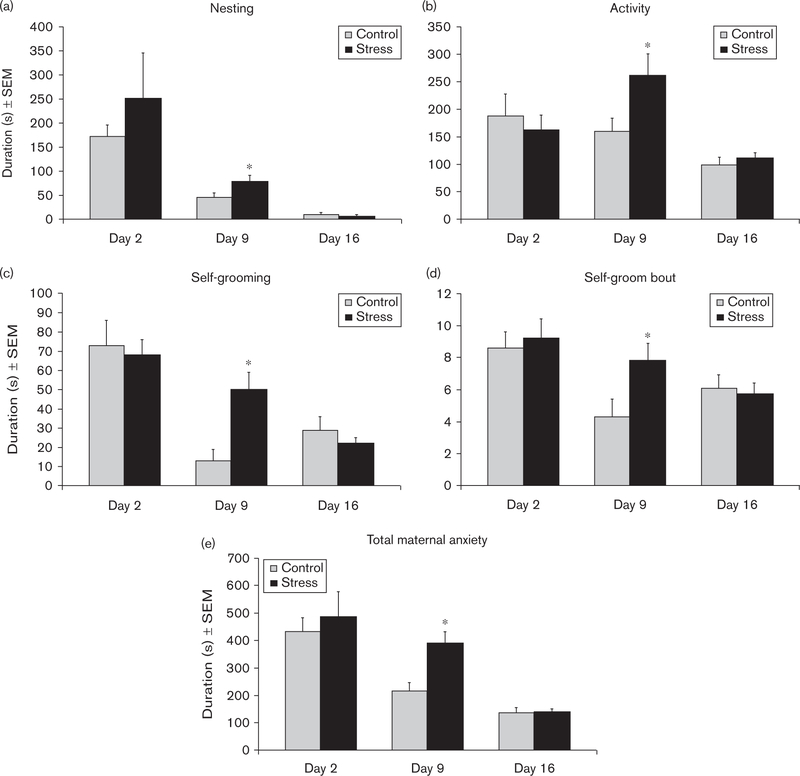
The durations of nonpup-directed behaviors increased on day 9 in CSS-exposed dams. The durations of nesting (F1,23=5.3, P<0.05; Fig. 2a), locomotor activity (F1,23=4.4, P<0.05; Fig. 2b), and self-grooming (F1,23=9.8, P<0.01; Fig. 2c), and the mean self-grooming bout duration (F1,23=5.0, P<0.05; Fig. 2d) were all elevated during the maternal care test on day 9. Instead of nursing and grooming the pups, the CSS dams were relocating their nest, grooming themselves, or patrolling the cage, resulting in a significant increase in maternal anxiety when the durations were combined (F1,23=11.0, P<0.01; Fig. 2e).
Fig. 2.
Mean duration ± SEM of nesting, locomotor activity, self-grooming, self-grooming bout duration, and total maternal anxiety on lactation days 2, 9, and 16. *Significant effect of CSS on that lactation day, P < 0.05. CSS, chronic social stress.
Milk intake and caloric density
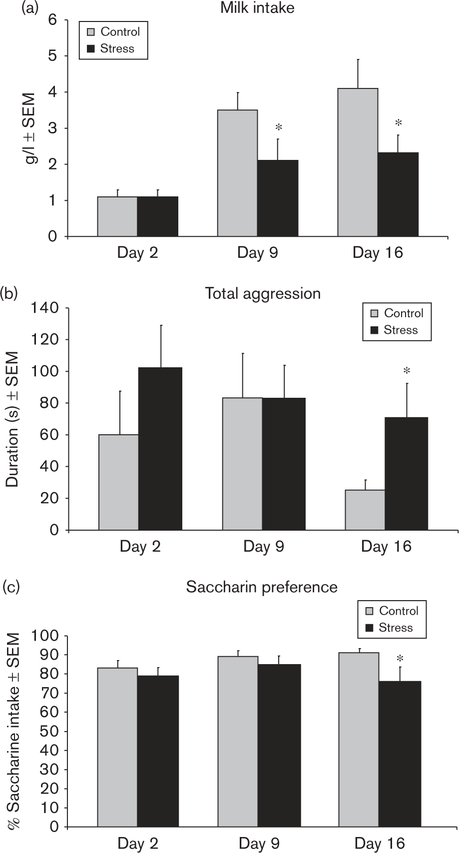
Milk intake by the pups of control and CSS dams did not significantly differ on day 2 before the start of the CSS protocol (P > 0.8), but it was significantly attenuated (40% lower) in the pups of CSS dams on lactation days 9 (F1,23 = 4.4, P < 0.05) and 16 (F1,23 = 4.5, P < 0.05; Fig. 3a). The caloric density of the combined milk samples from days 9 and 16 was 7277 calories/g for the control dams and 7245 calories/g for the CSS dams, a difference of 0.4%.
Fig. 3.
(a) The mean milk intake by the pups of CSS dams was decreased on lactation days 9 and 16 compared with control dams. (b) The mean maternal aggression duration displayed by CSS dams was increased on day 16. (c) The mean percentage of saccharin intake was decreased in CSS dams on lactation day 16. *Significant effect of CSS on that lactation day, P < 0.05. CSS, chronic social stress.
Maternal aggression
There were no significant differences in maternal aggression on days 2 and 9 (P’s > 0.3; Fig. 4). However, CSS dams were more aggressive on day 16 compared with control dams. CSS increased the duration of maternal aggression by 180% during late lactation (F1,23 = 4.5, P < 0.05; Fig. 3b).
Saccharin preference
There were no significant differences in total fluid intake on lactation days 2, 9, and 16. (P’s > 0.2). Saccharin preference was similar in the two groups on days 2 and 9 of lactation. On day 16, CSS dams exhibited an attenuated preference (F1,20 = 4.4, P < 0.05; Fig. 3c).
Gene expression
In the MeA, exposure to CSS decreased Oxt mRNA levels (F1,23 = 4.4, P < 0.05; Fig. 4a), whereas Crh mRNA expression increased in CSS dams in the CeA (F1,23 = 4.5, P < 0.05; Fig. 4b). There were no significant differences in the amygdalar gene expression levels of Avp, Avp1a, OxtR, Orx2R, Prl, GR, or mineralocorticoid receptor (MR). GR mRNA was increased in the PVN of CSS dams (F1,23 = 5.7, P < 0.05; Fig. 4c), whereas there were no significant differences in the mRNA expression levels of Avp, Avp1a, Oxt, Oxtr, Orx1R, Orx2R, MR, or Crh. Orx2R mRNA expression was elevated in the SON of CSS dams (F1,23 = 4.3, P < 0.05; Fig. 4d), with no significant changes in the expression levels of Avp, Avpr1a, Oxt, Oxtr, Orx1r, GR, MR, Prl, or Crh.
DNA methylation
DNA methylation was investigated in the PVN, focusing on a promoter region of the Nr3c1 gene coding for GR. The three CpGs studied span a previously described NGF1-A binding site (C(1)CGCCCC(2)CGCCCAGCTC(3)CG), with CpG2 being specifically regulated in a rat model of maternal care (Weaver et al., 2004). In the PVN DNA of CSS dams, methylation at CpGs 1 and 3 did not differ significantly, but CpG2 showed significantly reduced methylation (Fig. 4e) that was negatively correlated with PVN GR expression (r = − 0.43, r2 = 0.18, P < 0.05, one-tailed t-test).
Correlations
There was a significant gene–gene correlation between PVN GR and SON Orx2r (r = 0.71, r2 = 0.5, P < 0.01). There were significant gene–behavior correlations between MeA Oxt and day 9 total maternal care duration, and PVN GR, CeA Crh, and SON Orx2r and maternal anxiety (Table 1).
Table 1.
Gene–behavior correlations
| Gene | Nucleus | Day 9 behavior | r | R2 | P-value |
|---|---|---|---|---|---|
| OXT | MeA | Maternal care | 0.46 | 0.21 | 0.04 |
| CRH | CeA | Maternal anxiety | 0.48 | 0.23 | 0.04 |
| GR | PVN | Maternal anxiety | 0.46 | 0.21 | 0.03 |
| ORX2R | SON | Maternal anxiety | 0.52 | 0.27 | 0.02 |
Bold is used to highlight statistical significance.
CeA, central amygdale; CRH, corticotrophin-releasing hormone; GR, glucocorticoid receptor; MeA, medial amygdale; ORX, orexin; OXT, oxytocin; PVN, paraventricular nucleus; SON, supraoptic nucleus.
Endocrinology
There were no significant differences in basal plasma levels of corticosterone, estradiol, prolactin, or oxytocin between the control and CSS dams on day 23 (Table 2).
Table 2.
Basal plasma hormone levels
| Hormone | Control | CSS | P-value |
|---|---|---|---|
| Corticosterone | 91.1 ± 25.1 | 135.2 ± 34.1 | 0.23 |
| Estradiol | 27.1 ± 2.2 | 27.6 ± 3.0 | 0.90 |
| Prolactin | 35.7 ± 6.9 | 31.9 ± 8.0 | 0.72 |
| Oxytocin | 272.2 ± 40.5 | 265.5 ± 43.9 | 0.90 |
CSS, chronic social stress.
Discussion
The current study strongly supports and expands on previous data indicating that CSS is an ethologically relevant model for disorders that impair maternal care and lactation and attenuate offspring growth (Nephew and Bridges, 2011). Exposure to social stress depresses maternal care, impairs lactation, increases maternal anhedonia and anxiety, and induces a mild impairment in growth. CSS induced anhedonia on days 9 and 16, with maternal-specific anhedonia on day 9 and saccharin-based anhedonia on day 16, and substantial impairments in lactation were also present at both time points. These effects were associated with significant changes in gene expression of the neuropeptides Oxt and Crh, as well as GR and Orx2r.
Typical rodent maternal care involves retrieving all pups back to a well-formed nest, self-grooming by the dam, grooming of the pups, and then a substantial nursing bout. As expected, there were no differences between the two groups in maternal care on day 2 before the start of the CSS protocol. CSS dams spent more time gathering their pups to the nest and less time grooming and nursing the pups on day 9, following 1 week of social stress, supporting initial work on the CSS model (Nephew and Bridges, 2011). The increase in pup retrieval duration often involved circling the cage while carrying the pups and/or moving the nest location, and these actions were likely to affect other maternal care behaviors such as grooming and nursing, which are usually expressed after the pups are gathered in the nest. It is observed that this change in retrieval, and all changes in maternal behavior in the CSS dams, occurred in the absence of the male intruder and over 20 h after the most recent male intruder exposure. Maternal care is known to be highly rewarding to the dam (Mattson et al., 2001, 2003; Ferris et al., 2005; Ferris, 2014), and it is postulated that CSS disrupts the normal reward system in the brains of CSS dams, rendering the care of pups as less rewarding and decreasing maternal care and/or other measures of hedonia, similar to related stress-based rodent models of postpartum psychiatric illness (Smith et al., 2004; Brummelte et al., 2006; Brummelte and Galea, 2010).
In the CSS dams, the general progression of maternal behaviors from retrieval to grooming and nursing was often disrupted; they would start to retrieve some pups, briefly groom or nurse, then relocate the nest or retrieve other pups, resulting in substantial decreases in pup grooming and nursing. Nest relocation was rarely observed in control dams. Grooming during early and mid lactation impacts both physical and behavioral development in rodents (Champagne et al., 2003), and recent evidence has suggested a similar role in humans (Sharp et al., 2012). Impaired maternal care is a particularly adverse effect of PPD/A, which can exacerbate depression in the mother and mediate the development of mood and anxiety disorders in the offspring (Goodman et al., 2011). The decreased grooming by the CSS F0 mothers may mediate the robust neuroendocrine, endocrine, and behavioral changes documented in the F1 offspring (Carini and Nephew, 2013; Murgatroyd and Nephew, 2013). The pattern of adverse CSS effects on maternal care on day 9 but not day 16 supports the initial study of the CSS model (Nephew and Bridges, 2011). Given the fact that clinical definitions of PPD/A are based on depression during the first 4 weeks (DSM-IV) or the first 6 months (DSM-V) postpartum, and research on PPD/A is typically focused on diagnosis at a single time point during the first few months postpartum (O’Hara and McCabe, 2013; O’Hara and Wisner, 2014), the lack of effect of CSS on maternal care on day 16 is not a weakness of the model. Furthermore, although maternal care is not impaired on day 16, the saccharin preference data indicate anhedonia at this stage. In addition, related work on maternal care in the F1 and F2 offspring supports the hypothesis that CSS has maladaptive transgenerational effects on offspring despite the lack of effects on maternal care during late lactation (Carini and Nephew, 2013; Murgatroyd and Nephew, 2013; Babb et al., 2014). On the basis of early rodent studies, it is postulated that the dams may be responding to increased cues from the pups on day 16, such as vocalizations in response to the absence of the dam, decreased body temperature, and/or hunger (Smotherman et al., 1974; Henning and Romano, 1982; Cox et al., 2012; Okabe et al., 2013).
The decrease in maternal care on lactation day 9 was accompanied by an increase in maternal anxiety, and depression and anxiety are often comorbid (60–70%) in human mothers (Stuart et al., 1998; Sutter-Dallay et al., 2004; Reck et al., 2008; Wisner et al., 2013). As with depression, anxiety symptoms are often episodic during the postpartum period (O’Hara and Wisner, 2014). Instead of caring for the pups at a level similar to that in control dams on day 9, the CSS dams spent more time nesting, self-grooming, and moving around the cage, which is evidence of an overall increase in maternal anxiety (Kalueff and Tuohimaa, 2005). These results support findings in both dams exposed to CSS and their F1 offspring (Nephew and Bridges, 2011; Carini and Nephew, 2013). The changes in pup retrieval locomotion during maternal care testing are similar to the restlessness/ agitation and difficulties with decision-making that have been identified as specific features of PPD compared with major depression (Jolley and Betrus, 2007; Bernstein et al., 2008), and support the observations that depressed mothers exhibit a decrease in infant-directed focus and an increase in self-focus (Beebe et al., 2008; Stein et al., 2009). Further specific cognitive testing is needed to confirm the adverse effects of CSS on decision-making.
Although nursing duration was only decreased on day 9 in CSS dams, milk intake by the pups was substantially decreased on both days 9 and 16. Similar findings have been reported in F1 offspring of CSS dams (Carini and Nephew, 2013; Murgatroyd and Nephew, 2013), supporting the hypothesis that maternal care and lactation are mediated by a common mechanism (Stuebe et al., 2011). In humans, lactational difficulties are often associated with PPD/A (Dennis and McQueen, 2009; Stuebe et al., 2011, 2014; Watkins et al., 2011; Haga et al., 2012; Figueiredo et al., 2013), and impaired lactation can be mediated by the stress response (Lau, 1992, 2001; Lau and Simpson, 2004). Our initial analysis of the caloric density of the milk indicated that the CSS dams do not compensate for the impaired lactation through a substantial increase in caloric density. Furthermore, we found only a minor effect on pup growth, comparable to our earlier findings (Nephew and Bridges, 2011), where the dam also had continuous access to food and water. PPD/A in developed populations is not associated with severe developmental and growth effects, in contrast to developing populations in which access to food is restricted and substantial PPD-associated deficits in growth occur (Adewuya et al., 2008).
Similar to previous findings (Nephew and Bridges, 2011) CSS dams were more aggressive than controls on day 16 and did not show the usual decrease in maternal aggression during late lactation. This may be a sensitization response to the increased exposure to a threatening stimulus, which is central to this CSS model (Nephew and Bridges, 2011). It should be noted that this increase in maternal aggression is not required for effective protection of the pups, as there were no attacks on either the control or the CSS litters on day 16. The behavioral and neuroendocrine data suggest that CSS alters the neuroendocrine control of maternal aggression during lactation. Maternal aggression is strongly mediated by central changes in Crh activity in rodents (Gammie et al., 2004, 2008; D’Anna and Gammie, 2009). The CeA Crh increase supports prior work in highly aggressive multiparous females that have elevated central CRH levels compared with less aggressive primiparous dams (Nephew et al., 2009). Another potential mediator of the increase in maternal aggression in CSS dams is the decrease in MeA Oxt expression. Early-life maternal separation stress accentuates the later display of maternal aggression, and this is associated with decreased PVN Oxt expression (Veenema et al., 2007). Furthermore, PVN Oxt mRNA levels are decreased in multiparous rat dams, which show much higher levels of aggression when compared with primiparous dams (Nephew et al., 2009), supporting earlier reports of an inverse relationship between PVN OXT and maternal aggression (Giovenardi et al., 1997, 1998). Whereas the regions in which OXT levels are decreased differ between these studies, this may be due to significant differences between animal models.
Oxytocin neurons within the CeA and MeA are robust mediators of maternal care and social recognition in rodents (Bosch and Neumann, 2012; Nephew, 2012). We found a decrease in MeA Oxt expression to be specifically associated with maternal care, and not with maternal anxiety. Additional work with the CSS model in the F1 dams has strongly implicated OXT in the adverse effects of early-life CSS on maternal care (Murgatroyd and Nephew, 2013), and in the F2 offspring, deficient social behavior is accompanied by changes in plasma OXT levels (Babb et al., 2014). These and many other animal studies on female social behavior (Campbell, 2008; Bosch and Neumann, 2012) support recent clinical work indicating that OXT activity mediates human social behavior (Meyer-Lindenberg et al., 2011) and is altered in depressed females and mothers (Cyranowski et al., 2008; Ozsoy et al., 2009; Parker et al., 2010; Skrundz et al., 2011).
The observed increases in PVN GR and CeA Crh in CSS dams indicate an increased exposure to corticosterone during lactation, mediating the changes in maternal anxiety (Makino et al., 1994; Herman and Cullinan, 1997; Shepard et al., 2000). A study of nonstressed animals has reported stable levels of GR binding in the PVN across lactation (Meaney et al., 1989); therefore, GR and Crh could have been upregulated as a result of elevated plasma corticosterone levels from the repeated stressor, mediating the behavioral adaptation observed on day 16 when maternal anxiety was no longer elevated relative to control dams. It has been postulated that the increase in Orx2r mediated the stress-induced changes in CeA Crh and maternal anxiety (Sakamoto et al., 2004). The increase in GR and Crh may have allowed for maternal care similar to that in the control animals on day 16 either through a direct neuroendocrine effect or through a decrease in peripheral corticosterone levels (Brummelte and Galea, 2010). The correlations between GR and Crh expressions and maternal anxiety support the hypothesis that CSS induced elevated corticosterone levels, as exogenous corticosterone increases anxiety-related behaviors in nulliparous and maternal rats, and amygdalar CRH has an anxiogenic role (Swiergiel et al., 1993; Makino et al., 1994). The increase in CeA Crh expression supports previous work on CRH and self-grooming, as inhibition of CRH in the nucleus attenuated the corticosterone response to behavioral stress and decreased self-grooming (Callahan et al., 2013). A related hypothesis is that the decreased maternal care and increased maternal anxiety in CSS dams on day 9 was mediated by decreased OXT in the MeA and increased peripheral glucocorticoid levels. By day 16, the increased PVN GR and CeA Crh expressions in CSS dams may have decreased corticosterone exposure, allowing for maternal care levels similar to those in controls.
Maternal care in rats is associated with changes in DNA methylation levels at a specific CpG lying in an NGF1-A element within the exon 1–7 promoter region of the GR gene in the hippocampus. Specifically, offspring receiving high levels of licking and grooming have lower DNA methylation and reduced stress responsivity (Weaver et al., 2004, 2005). Further studies have linked maternal care to similar methylation changes in the hippocampus, cerebellum (Kosten and Nielsen, 2014), and hypothalamus (Mueller and Bale, 2008). We found reduced DNA methylation in the PVN at the same CpG that correlates with GR expression, adding to the growing literature demonstrating links between early-life environment (McGowan et al., 2009) and exposure to maternal depression (Oberlander et al., 2008), with altered DNA methylation at this gene.
The orexin (also known as hypocretin) system has been implicated in the pathophysiology of depression (Nollet and Leman, 2013) because of its putative involvement in behavioral control of multiple systems, including arousal, sleep/wake cycles, feeding, stress responses, and reward (Di Sebastiano and Coolen, 2012; Li et al., 2014), and in depression-associated hypothalamic changes in rodent models of depression (Nocjar et al., 2012). These systems are frequently disrupted in individuals with depression, and we report hypothalamic changes and disrupted reward-mediated maternal care in CSS dams. Orexin neurons, although confined primarily to the lateral hypothalamus, project to virtually every region of the brain except the cerebellum, and can have wide-reaching effects because of the ubiquitous distribution of its two receptors Orx1R and Orx2R. Studies in mice have implicated central orexin activity in the control of maternal care and maternal aggression (D’Anna and Gammie, 2006). The expression of this gene was correlated with maternal anxiety behavior in the current study (nesting, nonmaternal activity, and self-grooming), and it is postulated that the CSS-induced increase in central orexin activity mediated the decrease in maternal care through increased maternal anxiety. Changes in orexin are also associated with exposure to early-life stress and maternal deprivation (e.g. Feng et al., 2007), suggesting that orexin may mediate the reported transgenerational effects of early-life CSS to the F1 offspring.
Finally, the lack of differences in peripheral hormones is not surprising, given the timing of sample collection on lactation day 23, the evidence of HPA axis habituation to the repeated stressor, and the fact that the stressor was not applied until both populations had fully developed HPA and hypothalamic-pituitary-gonadal axes. Although there is some evidence that changes in hormone levels may play a role in the etiology of PPD in specific populations (Bloch et al., 2000; Meltzer-Brody et al., 2011; Workman et al., 2012), levels of estrogen, progesterone, and cortisol are similar in nondepressed and depressed postpartum women (Bloch et al., 2003; Workman et al., 2012). It is possible that there were differences during midlactation that were not present by day 23, and that the increases in GR and Crh are indicative of neural responses to the social stressor. The challenge and risk of studies with more extensive endocrine sampling are the introduction of an additional stressor in the paradigm and/or the loss of longitudinal strengths.
Conclusion
The data support the hypothesis that social stress during lactation can not only have profound effects on the ability of a dam to provide care to her offspring, it can also induce other relevant biomarkers of psychopathology, such as impaired lactation and increased maternal anxiety and restlessness. The behavioral changes induced by CSS are correlated with changes in specific neuropeptide systems in the brain of CSS dams, which may be epigenetically mediating the behavioral effects of social stress during this critical period. Future experiments should be targeted at understanding whether these changes in central gene expression are preceded by, or are causal factors of, the potent behavioral effects observed in this model.
Acknowledgements
This work was supported by NICHD grant R00 HD059943 and National Center for Advancing Translational Sciences, National Institutes of Health, grant UL1TR001064 to B.C.N.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Adewuya AO, Ola BO, Aloba OO, Mapayi BM, Okeniyi JA (2008). Impact of postnatal depression on infants’ growth in Nigeria. J Affect Disord 108:191–193. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH (2013). Depressive behavior and activation of the orexin/hypocretin system. Behav Neurosci 127:86–94. [DOI] [PubMed] [Google Scholar]
- Babb JA, Carini LM, Spears SL, Nephew BC (2014). Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm Behav 65:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe B, Jaffe J, Buck K, Chen H, Cohen P, Feldstein S, Andrews H (2008). Six-week postpartum maternal depressive symptoms and 4-month mother–infant self- and interactive contingency. Infant Ment Health J 29:442–471. [DOI] [PubMed] [Google Scholar]
- Bernstein IH, Rush AJ, Yonkers K, Carmody TJ, Woo A, McConnell K, Trivedi MH (2008). Symptom features of postpartum depression: are they distinct? Depress Anxiety 25:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettscheider M, Murgatroyd C, Spengler D (2011). Simultaneous DNA and RNA isolation from brain punches for epigenetics. BMC research notes 4:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR (2000). Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 157:924–930. [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR (2003). Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 44:234–246. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID (2012). Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav 61:293–303. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA (2010). Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav 58:769–779. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Pawluski JL, Galea LA (2006). High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: A model of post-partum stress and possible depression. Horm Behav 50:370–382. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Rigero BA, Bridges RS (2000). Opioid receptor antagonism during early lactation results in the increased duration of nursing bouts. Physiol Behav 70:211–216. [DOI] [PubMed] [Google Scholar]
- Callahan LB, Tschetter KE, Ronan PJ (2013). Inhibition of corticotropin releasing factor expression in the central nucleus of the amygdala attenuates stress-induced behavioral and endocrine responses. Front Neurosci 7:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A (2008). Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biol Psychol 77:1–10. [DOI] [PubMed] [Google Scholar]
- Carini LM, Nephew BC (2013). Effects of early life social stress on endocrinology, maternal behavior, and lactation in rats. Horm Behav 64:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini LM, Murgatroyd CA, Nephew BC (2013). Using chronic social stress to model postpartum depression in lactating rodents. J Vis Exp e50324. [DOI] [PMC free article] [PubMed]
- Champagne FA, Francis DD, Mar A, Meaney MJ (2003). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 79:359–371. [DOI] [PubMed] [Google Scholar]
- Cox ET, Hodge CW, Sheikh MJ, Abramowitz AC, Jones GF, Jamieson-Drake AW, et al. (2012). Delayed developmental changes in neonatal vocalizations correlates with variations in ventral medial hypothalamus and central amygdala development in the rodent infant: effects of prenatal cocaine. Behav Brain Res 235:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA (2008). Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med 70:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna KL, Gammie SC (2006). Hypocretin-1 dose-dependently modulates maternal behaviour in mice. J Neuroendocrinol 18:553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna KL, Gammie SC (2009). Activation of corticotropin-releasing factor receptor 2 in lateral septum negatively regulates maternal defense. Behav Neurosci 123:356–368. [DOI] [PubMed] [Google Scholar]
- Dennis CL, McQueen K (2009). The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics 123:e736–e751. [DOI] [PubMed] [Google Scholar]
- Di Sebastiano AR, Coolen LM (2012). Orexin and natural reward: feeding, maternal, and male sexual behavior. Prog Brain Res 198:65–77. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322:900–904. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, et al. (2011). Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev Psychopathol 23:1039–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E (2009). Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry 48:919–927. [DOI] [PubMed] [Google Scholar]
- Feng P, Vurbic D, Wu Z, Strohl KP (2007). Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain Res 18:163–172. [DOI] [PubMed] [Google Scholar]
- Ferris C (2014). Using awake animal imaging to understand neural circuits of emotion: studies ranging from maternal care to aggression. In: Decety J, Christen Y, editors. New Frontiers in Social Neuroscience New York, NY: Springer International Publishing; pp. 111–126. [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM Jr, Harder JA, Messenger TL, Febo M (2005). Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci 25:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo B, Canário C, Field T (2014). Breastfeeding is negatively affected by prenatal depression and reduces postpartum depression. Psychol Med 44:927–936. [DOI] [PubMed] [Google Scholar]
- Forman DR, O’Hara MW, Stuart S, Gorman LL, Larsen KE, Coy KC (2007). Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol 19:585–602. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS (2004). Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci 118:805–814. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Seasholtz AF, Stevenson SA (2008). Deletion of corticotropin-releasing factor binding protein selectively impairs maternal, but not intermale aggression. Neuroscience 157:502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB (1997). Hypothalamic para-ventricular nucleus, oxytocin, and maternal aggression in rats. Ann N Y Acad Sci 807:606–609. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB (1998). Hypothalamic para-ventricular nucleus modulates maternal aggression in rats: effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav 63:351–359. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D (2011). Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 14:1–27. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE (2003). The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Womens Ment Health 6:263–274. [DOI] [PubMed] [Google Scholar]
- Gress-Smith JL, Luecken LJ, Lemery-Chalfant K, Howe R (2012). Postpartum depression prevalence and impact on infant health, weight, and sleep in low-income and ethnic minority women and infants. Matern Child Health J 16:887–893. [DOI] [PubMed] [Google Scholar]
- Haga SM, Ulleberg P, Slinning K, Kraft P, Steen TB, Staff A (2012). A longitudinal study of postpartum depressive symptoms: multilevel growth curve analyses of emotion regulation strategies, breastfeeding self-efficacy, and social support. Arch Womens Ment Health 15:175–184. [DOI] [PubMed] [Google Scholar]
- Henning SJ, Romano TJ (1982). Investigation of body temperature as a possible feeding control in the suckling rat. Physiol Behav 28:693–696. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE (1997). Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ (2001). The neurobiology of attachment. Nat Rev Neurosci 2:129–136. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM (2011). Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry 2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley SN, Betrus P (2007). Comparing postpartum depression and major depressive disorder: issues in assessment. Issues Ment Health Nurs 28:765–780. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P (2005). The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J Neurosci Methods 143:169–177. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Nielsen DA (2014). Litter and sex effects on maternal behavior and DNA methylation of the Nr3c1 exon 17 promoter gene in hippocampus and cerebellum. Int J Dev Neurosci 36:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C (1992). Effects of various stressors on milk release in the rat. Physiol Behav 51:1157–1163. [DOI] [PubMed] [Google Scholar]
- Lau C (2001). Effects of stress on lactation. Pediatr Clin North Am 48:221–234. [DOI] [PubMed] [Google Scholar]
- Lau C, Simpson C (2004). Animal models for the study of the effect of prolonged stress on lactation in rats. Physiol Behav 82:193–197. [DOI] [PubMed] [Google Scholar]
- Li J, Hu Z, de Lecea L (2014). The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol 171:332–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J (1994). Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res 640:105–112. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI (2001). Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci 115:683–694. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI (2003). Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology (Berl) 167:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Viau V, Aitken DH, Bhatnagar S (1989). Glucocorticoid receptors in brain and pituitary of the lactating rat. Physiol Behav 45:209–212. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Stuebe A, Dole N, Savitz D, Rubinow D, Thorp J (2011). Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD). J Clin Endocrinol Metab 96:E40–E47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12:524–538. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL (2008). Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci 28:9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Nephew BC (2013). Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology 38:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC. Behavioral roles of oxytocin and vasopressin. In: Sumiyoshi T, editor. Neuroendocrinology and behavior Rijeka, Croatia: InTech; 2012. [Google Scholar]
- Nephew BC, Bridges RS (2011). Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress 14:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM (2009). Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behav Neurosci 123:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, Panksepp J (2012). The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience 218:138–153. [DOI] [PubMed] [Google Scholar]
- Nollet M, Leman S (2013). Role of orexin in the pathophysiology of depression: potential for pharmacological intervention. CNS Drugs 27:411–422. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, McCabe JE (2013). Postpartum depression: current status and future directions. Annu Rev Clin Psychol 9:379–407. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Wisner KL (2014). Perinatal mental illness: definition, description and aetiology. Best Pract Res Clin Obstet Gynaecol 28:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM (2008). Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 3:97–106. [DOI] [PubMed] [Google Scholar]
- Okabe S, Nagasawa M, Kihara T, Kato M, Harada T, Koshida N, et al. (2013). Pup odor and ultrasonic vocalizations synergistically stimulate maternal attention in mice. Behav Neurosci 127:432–438. [DOI] [PubMed] [Google Scholar]
- Ozsoy S, Esel E, Kula M (2009). Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res 169:249–252. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, Schatzberg AF (2010). Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res 178:359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RM, Evans J, Kounali D, Lewis G, Heron J, Ramchandani PG, et al. (2013). Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry 70:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani CV, Slattery DA (2014). Using animal models to study postpartum psychiatric disorders. Br J Pharmacol 16:12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck C, Struben K, Backenstrass M, Stefenelli U, Reinig K, Fuchs T, et al. (2008). Prevalence, onset and comorbidity of postpartum anxiety and depressive disorders. Acta Psychiatr Scand 118:459–468. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y (2004). Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic para-ventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept 118:183–191. [DOI] [PubMed] [Google Scholar]
- Sharp H, Pickles A, Meaney M, Marshall K, Tibu F, Hill J (2012). Frequency of infant stroking reported by mothers moderates the effect of prenatal depression on infant behavioural and physiological outcomes. PLoS One 7: e45446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA (2000). Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 861:288–295. [DOI] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G (2011). Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology 36:1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW (2004). Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology 29:227–244. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA (1974). Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol 12:55–66. [DOI] [PubMed] [Google Scholar]
- Sohr-Preston SL, Scaramella LV (2006). Implications of timing of maternal depressive symptoms for early cognitive and language development. Clin Child Fam Psychol Rev 9:65–83. [DOI] [PubMed] [Google Scholar]
- Stein A, Lehtonen A, Harvey AG, Nicol-Harper R, Craske M (2009). The influence of postnatal psychiatric disorder on child development. Is maternal preoccupation one of the key underlying processes? Psychopathology 42:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart S, Couser G, Schilder K, O’Hara MW, Gorman L (1998). Postpartum anxiety and depression: onset and comorbidity in a community sample. J Nerv Ment Dis 186:420–424. [DOI] [PubMed] [Google Scholar]
- Stuebe AM, Grewen KM, Pedersen CA, Propper C, Meltzer-Brody S (2011). Failed lactation and perinatal depression: common problems with shared neuroendocrine mechanism. J Womens Health (Larchmt) 21:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Horton BJ, Chetwynd E, Watkins S, Grewen K, Meltzer-Brody S (2014). Prevalence and risk factors for early, undesired weaning attributed to lactation dysfunction. J Womens Health (Larchmt) 23:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter-Dallay AL, Giaconne-Marcesche V, Glatigny-Dallay E, Verdoux H (2004). Women with anxiety disorders during pregnancy are at increased risk of intense postnatal depressive symptoms: a prospective survey of the MATQUID cohort. Eur Psychiatry 19:459–463. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Takahashi LK, Kalin NH (1993). Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res 623:229–234. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID (2007). Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology 32:437–450. [DOI] [PubMed] [Google Scholar]
- Villegas L, McKay K, Dennis CL, Ross LE (2011). Postpartum depression among rural women from developed and developing countries: a systematic review. J Rural Health 27:278–288. [DOI] [PubMed] [Google Scholar]
- Watkins S, Meltzer-Brody S, Zolnoun D, Stuebe A (2011). Early breastfeeding experiences and postpartum depression. Obstet Gynecol 118 (2 Pt 1): 214–221. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR (2004). Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M (2005). Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25:11045–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, et al. (2013). Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry 70:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Barha CK, Galea LA (2012). Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci 126:54–72. [DOI] [PubMed] [Google Scholar]
- Young LJ (1999). Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Behav 36:212–221. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ (2013). Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology 38:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]