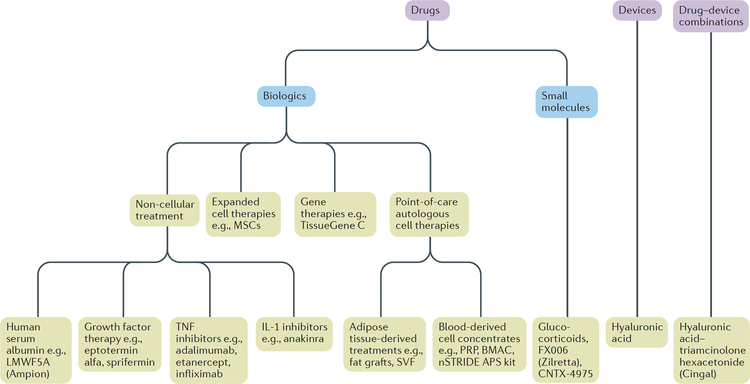
Figure 1. Intra-articular treatments for osteoarthritis.
Intra-articular treatments for osteoarthritis are approved by the FDA as drugs, devices or drug-device combination products. Drugs are classified as small molecules (<900 Daltons) or biologics, which can be further broken down into four sub-categories (non-cellular therapies, expanded cell therapies, gene therapies and point-of-care autologous cell therapies). The therapeutic effects of non-cellular biologic drugs depend on single large complex molecules or on specific mixtures of molecules. For the sake of simplicity, only clinically investigated human serum albumin, TNF inhibitors, IL-1 inhibitors and growth factors are included. Expanded cell therapies are biologic drug ‘factories’ that are subject to strict regulatory oversight, whereas gene therapies introduce genes that make beneficial protein(s) or compensate for abnormal genes. Point-of-care autologous cell therapies are heterogeneous mixtures containing cells (or cell products) that are derived from autologous blood, bone marrow or adipose tissue and are often given to patients off-label. APS, autologous protein solution; BMAC, bone marrow aspirate concentrate; MSC, mesenchymal stem cell; PRP, platelet-rich plasma; SVF, stromal vascular fraction.