Abstract
Welding fumes were reclassified as a Group 1 carcinogen by the International Agency for Research on Cancer in 2017. Gas metal arc welding (GMAW) is a process widely used in industry. Fume generated from GMAW-mild steel (MS) is abundant in iron with some manganese, while GMAW-stainless steel (SS) fume also contains significant amounts of chromium and nickel, known carcinogenic metals. It has been shown that exposure to GMAW-SS fume in A/J mice promotes lung tumors. The objective was to determine if GMAW-MS fume, which lacks known carcinogenic metals, also promotes lung tumors in mice. Male A/J mice received a single intraperitoneal injection of corn oil or the initiator 3-methylcholanthrene (MCA; 10 μg/g) and, one week later, were exposed by whole-body inhalation to GMAW-MS aerosols for 4 hours/day x 4 days/week x 8 weeks at a mean concentration of 34.5 mg/m3. Lung nodules were enumerated by gross examination at 30 weeks postinitiation. GMAW-MS fume significantly increased lung tumor multiplicity in mice initiated with MCA (21.86 ± 1.50) compared to MCA/air-exposed mice (8.34 ± 0.59). Histopathological analysis confirmed these findings and also revealed an absence of inflammation. Bronchoalveolar lavage analysis also indicated a lack of lung inflammation and toxicity after short-term inhalation exposure to GMAW-MS fume. In conclusion, this study demonstrates that inhalation of GMAW-MS fume promotes lung tumors in vivo and aligns with epidemiologic evidence that shows MS welders, despite less exposure to carcinogenic metals, are at an increased risk for lung cancer.
Keywords: Mild steel, Welding, Inhalation, Iron, A/J mice, Tumor promotion
1. Introduction
Welding fumes were recently reclassified as carcinogenic to humans (Group 1) by the International Agency for Research on Cancer (IARC) based on strong epidemiological evidence and limited evidence in animals (Guha et al., 2017). It is estimated that 11 million workers worldwide weld full-time and an additional 110 million have had some type of welding-related exposure (Guha et al., 2017). Arc welding, including one type known as gas metal arc welding (GMAW), is the most common industrial welding process (Antonini, 2014; The Procedure Handbook of Arc Welding, 2018). In GMAW, an electric arc is established between a work piece and a consumable wire electrode. High temperatures create a molten pool into which the electrode is continuously fed and the work pieces are fused together as temperatures cool. While this process is the strongest method of joining metals, it creates a significant amount of welding fume. The composition of the fume largely depends on whether a stainless steel (SS) or mild steel (MS) electrode is used. GMAW-SS fume contains largely iron (Fe), chromium (Cr), nickel (Ni), copper (Cu), and manganese (Mn), whereas GMAW-MS contains primarily Fe and Mn. Most experimental studies have focused on the presumably more toxic Cr and Ni and largely overlooked Fe when examining pulmonary toxicity and/or carcinogenicity of welding fume. Importantly, many epidemiological studies suggest that both SS and MS arc welding fumes are associated with increased risk of lung cancer, even though GMAW-MS fume exposure is predominantly limited to Fe and Mn (Hansen and Lauritsen, 1996; Lauritsen and Hansen, 1996). Some studies are conflicting, however (Sorensen et al., 2007). Welding exposures are complex because of the diversity of welding modalities used in the workplace and the potential for confounders or additional occupational exposures (Antonini, 2014; Matrat et al., 2016). Welders often perform multiple types of welding processes throughout their lifetime, further complicating epidemiological studies. Therefore, controlled animal studies are crucial to better understand which welding fumes and their component metals are the most toxic and have the greatest tumorigenic potential.
It was previously shown that GMAW-SS fume persists in the lung for 1.5 years and triggers mild, chronic inflammation in lung tumor-susceptible A/J mice compared to other welding fumes (Zeidler-Erdely et al., 2011). In a two-stage initiation-promotion model of lung tumorigenesis, GMAW-SS fume significantly increased lung tumor multiplicity after both an oropharyngeal aspiration and inhalation exposure in A/J mice (Falcone et al., 2017; Zeidler-Erdely et al., 2013). It was also demonstrated that GMAW-MS fume increased lung cytotoxicity after an oropharyngeal aspiration exposure (Zeidler-Erdely et al., 2008). However, GMAW-SS had a greater cytotoxic effect than GMAW-MS fume. Similarly, studies in rats have indicated that GMAW-MS seems to be less toxic than GMAW-SS fume (Antonini et al., 2009, 2007; Taylor et al., 2003). Antonini et al. found that GMAW-MS fume caused no lung inflammation or lung injury in Sprague-Dawley rats 1, 4, or 11 days post-inhalation compared to GMAW-SS fume which caused significant lung damage (Antonini et al., 2009, 2007).
It is well known that certain conditions associated with iron-overloaded states lead to an increased risk of cancer. While iron plays a vital role in redox reactions and as a cofactor for enzymatic reactions in the body, too much iron can increase cancer risk via the production of reactive oxygen species (Manz et al., 2016; Andrews, 2000). Asbestosis, hemochromatosis, mylodysplastic syndromes, and endometriosis are all diseases in which there is iron excess and increased risk of cancer (Akatsuka and Toyokuni, 2016; Steegmann-Olmedillas, 2011). Epidemiologic studies concerning iron oxide exposures and lung cancer are conflicting. An early study of iron ore miners found that these workers had a 70% greater mortality of lung cancer than the general population (Boyd et al., 1970). Yet, other reports suggest that iron oxide is not a human carcinogen (Pease et al., 2016; Bourgkard et al., 2009). An early in vivo study by Campbell in 1940 found an increase in lung carcinomas in mice exposed to iron oxide (Campbell, 1940). Regardless, in vivo studies investigating occupational exposures to iron oxides, as occurs with mild steel welding, are lacking. In this study, we aimed to characterize the lung toxicity of GMAW-MS, which contains primarily Fe, and determine if it could promote lung tumors in mice following inhalation exposure.
2. Methods
2.1. Animals
Male A/J mice (age 4–5 week) were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in an AAALAC International-specific pathogen-free, environmentally-controlled facility. All mice were free of endogenous pathogens including viruses, bacteria, mycoplasmas, and parasites. Mice were housed in groups of two in ventilated cages and provided high-efficiency particulate filtered air under a controlled light cycle (12 h light/12 h dark) at a standard temperature (22–24⁰C) and 30–70% relative humidity. Animals were acclimated to the animal facility for one week before beginning the experimental protocols and allowed access to a conventional diet (6% irradiated NIH-31 Diet, Envigo RMS, Inc.; Madison, WI) and tap water ad libitum. All procedures were performed using protocols approved by the National Institute for Occupational Safety and Health (NIOSH) Institutional Animal Care and Use Committee.
2.2. Welding fume inhalation exposure system
The design and construction of the welding fume aerosol generator were previously described (Antonini et al., 2006). This automated robotic welder continuously generated welding fumes by welding beads onto ¼ inch thick plates of mild steel. The welding wire used was 0.045 inch diameter Lincoln Electric Super Arc MIG L56 and the welding parameters were set to 25 V DC, 300 inch per minute wire feed, 30 l/min of 95% argon – 5% CO2 shielding gas, and a typical welding current of 220 amps. The resulting fume was carried into a whole body animal exposure chamber through a ¾ inch flexible tube by maintaining the chamber at a negative pressure (0.70 inch H2O). The chamber was constructed primarily of stainless steel with any openings covered with an anti-UV coating film. Particle concentrations within the exposure chamber were continuously monitored with a Data RAM (DR-40000 Thermo Electron Co; Franklin, MA), and gravimetric determinations (37 mm cassettes with 0.45 μm pore-size Teflon filters) were used to calibrate and verify the Data RAM readings each day. Gas generation, including carbon monoxide (CO), carbon dioxide (CO2), oxygen (O2), and ozone (O3), was continuously monitored during the exposures. Levels of O2 were maintained above the OSHA minimal acceptable level. O3, CO, CO2 were below OSHA permissible exposure limits and NIOSH recommended exposure limits (REL) during the entire exposure duration. In the exposure chamber, CO and O3 levels were not significantly higher than background. The exposure system was modified slightly from that described previously to reduce the travel time of the particulate fume from the welding torch to the exposure chamber (Antonini et al., 2006).
2.3. Welding fume metal analysis
A small amount of welding fume was collected gravimetrically onto 47-mm Nucleopore polycarbonate filters (Whatman; Clinton, PA) for field emission scanning electron microscopy (FESEM) to assess particle size and morphology. The particles were imaged using a Hitachi S4800 Field Emission Scanning Electron Microscope (Hitachi; Tokyo, Japan). For elemental analysis of GMAW-MS fume, generated particles were collected inside the exposure chamber onto 5.0 μm polyvinyl chloride membrane filters in 37-mm cassettes during three 30 min collections. The particle samples were digested and the metals were analyzed by inductively coupled plasma atomic emission spectroscopy according to the NIOSH method 7303 for hot block/HCL/HNO3 digestion (NIOSH, 1994) as previously described (Antonini et al., 2006). Metal content of blank filters also was analyzed for control purposes.
2.4. Experimental protocol for whole lung metal analysis
Weight-matched A/J mice were exposed by whole-body inhalation in individual steel mesh cages to GMAW-MS welding aerosols (mean concentration 36.4 mg/m3 over 4 hours) (n = 10) or filtered air (n = 10) (Fig. 1A). Immediately following exposure (time zero), whole lungs were excised, trimmed, and lyophilized. The freeze-dried tissue was weighed then acid digested. Inductively coupled argon plasma atomic emission spectroscopy at NIOSH-Division of Applied Research and Technology (Cincinnati, OH) was used to determine the amount of aluminum (Al), Cr, Cu, Fe, Mn, Ni, zinc (Zn) present in the lung according to the NIOSH method 7300 modified to accommodate the sample matrix (NIOSH 2003).
Fig. 1. Timelines of experimental protocols.
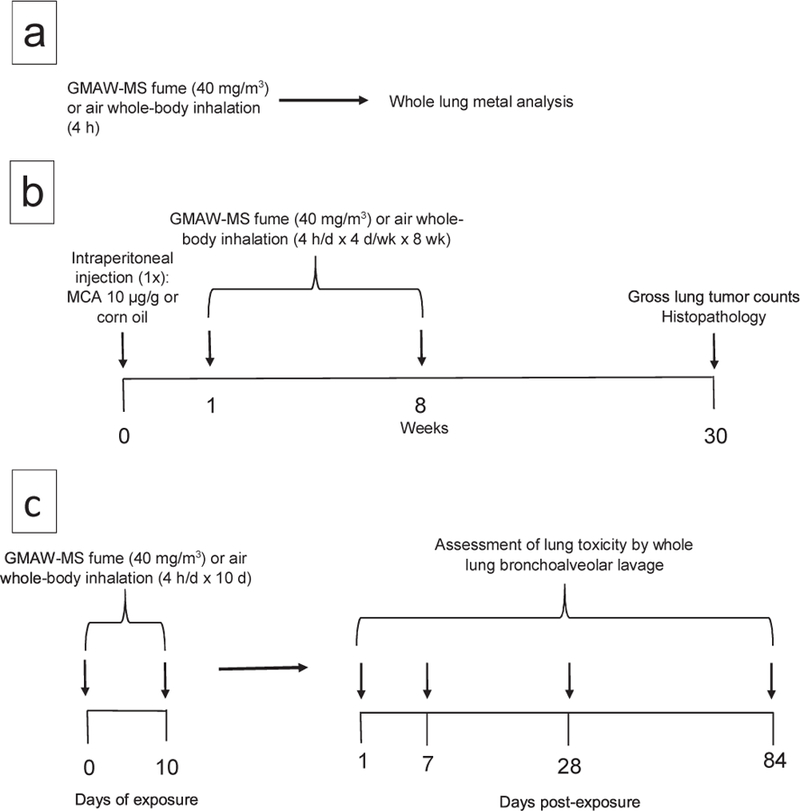
Panel A – Experimental protocol for whole-lung metal analysis. A/J mice were exposed by whole-body inhalation to filtered air (n = 10) or GMAW-MS fume (n = 10) for 4 hours. Immediately following the exposure, whole lungs were excised, trimmed, and lyophilized. Inductively coupled argon plasma, atomic emission spectroscopy was used to analyze the metal content of lungs. Panel B - Experimental protocol for two-stage (initiation-promotion) lung carcinogenesis bioassay. Mice received a single MCA or corn oil intraperitoneal injection then beginning 1 week later were exposed to GMAW-MS or air by whole-body inhalation for 4 hours/day x 4 days/week x 8 weeks before terminal sacrifice at 30 weeks (n=30/group). Panel C - Experimental protocol for whole-lung BAL toxicity assessment. A/J mice were exposed by whole-body inhalation to GMAW-MS fume or air for 4 hours/day for 10 days. BAL was done to assess lung injury and inflammation at different time points post-exposure (n = 8 per group per time point).
2.5. Experimental protocol for two-stage lung carcinogenesis bioassay in A/J mice
For the two-stage initiation-promotion protocol, 120 mice were weight-matched and randomized into four exposure groups (n = 30/ group). On day 1, mice were intraperitoneally (IP) injected one time with the chemical initiator, 3-methylcholanthrene (MCA) (Sigma-Aldrich; St. Louis, MO) dissolved in corn oil (CO) (Sigma-Aldrich; St. Louis, MO) at a dose of 10 μg/g of body weight or CO alone (Fig. 1B). MCA was chosen as the initiating agent based on the efficient response of the A/J mouse to this carcinogen in our previous oropharyngeal aspiration and inhalation studies (Zeidler-Erdely et al., 2013; Falcone et al., 2017). Beginning 1 week post-initiation, mice were exposed by whole-body inhalation to GMAW-MS aerosols or filtered air for 4 hours/ day, 4 days/week, for 8 weeks at a target concentration of 40 mg/m3 (actual mean concentration 34.5 mg/m3 over 8 weeks). Throughout the study, mice were weighed biweekly including at the terminal sacrifice at 30-weeks post-initiation. Mice were euthanized with sodium pento-barbital [100–300 mg/kg IP] (Vortech Pharmaceuticals; Dearborn, MI), weighed, and exsanguinated via the vena cava. All internal organs were examined for the presence of tumors. Then, the whole lung was excised and inflated with 10% neutral buffered formalin. Twenty-four hours post-fixation, lung tumors were counted. Lung tumor incidence was recorded as the percent of tumor-bearing mice out of the total. Lung tumor multiplicity was determined as the average tumor number per mouse lung including mice with no tumors. Any apparent merged tumors were counted as one tumor. Lungs were embedded in paraffin before a 5 μm standardized section was cut and slides were made.
Slides were stained with hematoxylin and eosin, and a contracted, board certified veterinary pathologist observed the slides in a blinded fashion for evidence of hyperplasia or neoplasia, inflammation, lymphoid tissue response, and foreign materials by light microscopy. The diagnosis of alveolar hyperplasia, adenoma, and adenocarcinoma was based upon well-established criteria (Renne et al., 2009). If abnormal changes were found, severity was scored as: 1 = minimal, 2 = mild, 3 = moderate, 4 = marked. Severity of hyperplasia was graded based upon the overall size of the lesion, and ranged from small foci, which were graded as minimal, to large foci, which were graded as marked. The severity of the hyperplasia was recorded as the severity of the most severe lesion seen in each lung. The final severity score reflects the average of the right and left lung lobe scores and is presented as means ± standard error. Because bronchioloalveolar hyperplasia (BAH), bronchioloalveolar adenomas (BAA), and bronchioloalveolar adenocarcinomas (BAC) represent a continuum of the proliferative process, and there is possible overlap between these diagnoses, the numbers of lesions were combined to compare the tumorigenic potential of each treatment (Renne et al., 2009). However, examination of a single histological section per lung underestimates the total number of lesions per lung, making the gross tumor count at time of sacrifice more indicative of the actual response (Rehm and Ward, 1989).
2.6. Experimental protocol for bronchoalveolar lavage (BAL) and biochemical measurements of lung toxicity
For the lung toxicity protocol, A/J mice were exposed to filtered air or GMAW-MS fume at a target concentration of 40 mg/m3 (actual mean concentration 36.28 mg/m3) for 4 hours/day for 10 days (Fig. 1C). Whole-lung BAL was used to assess lung injury and inflammation at 1, 7, 28, and 84 days post-exposure (n = 8 per group per time point). Mice were anesthetized with sodium pentobarbital (100–300 mg/kg IP; Vortech Pharmaceuticals) then weighed. Once unresponsive, 0the mouse was then exsanguinated via the vena cava. For BAL, the trachea was cannulated with a blunted 22 gauge needle and the thorax was massaged as 0.6 ml of cold calcium and magnesium-free phosphate buffered saline (PBS) was instilled into the lungs. The thorax was massaged for 10 seconds before the fluid was withdrawn and placed in a 15 ml conical tube. This consisted of the first lavage fraction. BAL was repeated 3 times using 1 ml of PBS per instillate and this second fraction was collected in a separate 15 ml conical tube. The BAL fluid was preserved on ice then centrifuged at 500 x g, 10 min, and 4 °C. The acellular supernatant of the first lavage fraction was used to measure lactate dehydrogenase (LDH) activity, indicative of lung cytotoxicity. LDH activity was analyzed using a COBAS MIRA Plus auto-analyzer (Roche Diagnostic Systems; Montclair, NJ) which measured the oxidation of lactate to pyruvate coupled with the formation of NADH at 340 nm. The supernatant from the second lavage fraction was discarded. The cell pellets of both fractions were combined and resuspended in 800 μl of PBS. This final cell pellet was used for cell enumeration and differentials. For cell enumeration, cells were gently vortexed then combined in a 1:2 dilution with trypan blue (Sigma-Aldrich). The suspension was slowly mixed with a pipette and 10 μl was loaded onto the hemocytometer. The number of live cells in the four outer squares were recorded and cell concentration was calculated as: total cell count in 4 squares x 2500 × 2 (dilution factor). For cell differentials, cells were plated onto glass slides using a Cytospin 3 centrifuge (Shandon Life Sciences International; Cheshire, England) set at 800 rpm for 5 min. Slides were stained with Hema 3 Fixative and Solutions (Fisher Scientific; Pittsburgh, PA) then cover slipped. A minimum of 300 cells/slide were identified using light microscopy.
2.7. Statistical comparisons and analysis
Statistical analyses were performed using either JMP version 13, or SAS version 9.4 for Windows. Continuous variables were analyzed using treatment by day factorial analyses of variance (ANOVA), followed by Fishers LSD for pairwise comparisons. For some variables, a natural log transformation was performed on the data to reduce heterogeneous variance and meet the assumptions of an ANOVA. Score variables such as hyperplasia severity were analyzed using nonparametric Kruskal-Wallis tests and followed by pair-wise comparisons using the Wilcoxon Rank Sums test. Gross tumor counts and histopathology count data from sections were analyzed similarly. Tumor incidence was analyzed using a Chi-square test in SAS ‘Proc Freq,’ while tumor multiplicity was analyzed using Poisson regression in SAS ‘Proc Genmod.’ In cases where over dispersion existed, a negative binomial regression was performed. Analyses were performed independently on CO and MCA-treated animals, and only utilized data from those animals surviving to the 30-week time point. For all analyses, a p-value of < 0.05 was set as the criteria for significance.
3. Results
3.1. Welding fume characteristics
Scanning electron microscope (SEM) images of GMAW-MS fume are presented in Fig. 2 and show the particles forming chain-like aggregates. GMAW-MS fume ranged in size from nanoparticles to larger, coarse particles with most particles between 0.1 to 1 μm in size. The mass median aerodynamic diameter (MMAD) was 0.31 μm. Elemental analysis indicated that the fume was primarily Fe and Mn (Table 1). Fe content by weight percent averaged 83.67% and Mn was 14.33%. Approximately 2% of the fume consisted of other trace metals.
Fig. 2. SEM images of GMAW-MS fume.
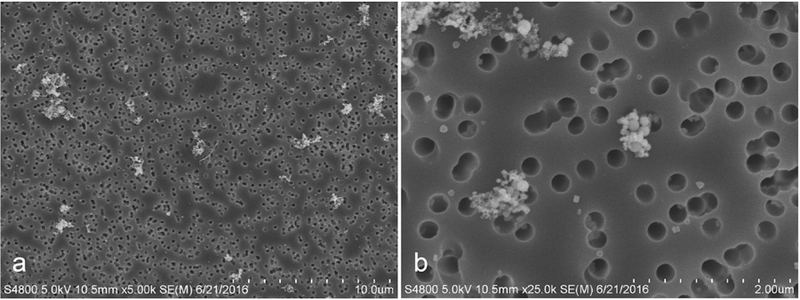
Particles form small clustered aggregates ranging in size from less than 0.01 μm to over 1 μm, with a mass median aerodynamic diameter of 0.31 μm (Antonini et al., 2009). Primary particles were mainly in the nanometer size range and primarily composed of iron (83.67%) and manganese (14.33%).
Table 1.
Metal composition of Gas Metal Arc Welding-Mild Steel fume. The fume was analyzed for aluminum (Al), chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), silicon (Si), titanium (Ti), vanadium (V), and zinc (Zn) by Inductively Coupled Plasma-Atomic Emission Spectroscopy. Samples were prepared according to the NIOSH method 7303 modified for microwave digestion. Trace amounts of Al, Cr, Ni, Si, Ti, and V were found. Note: Values are means ± standard error (n = 3 welding fume collection periods of 30 min). Weight % is relative to all metals analyzed.
| Metals | Fume Weight % of Metals |
|---|---|
| Fe | 83.67 ± 0.47 |
| Mn | 14.33 ± 0.37 |
| Cu | 0.13 ± 0.03 |
3.2. Whole lung metal deposition after GMAW-MS fume inhalation
The lung metal deposition in A/J mice measured at time 0 after 4 hours of inhalation of GMAW-MS fume is shown in Table 2 and was calculated as done previously (Falcone et al., 2017). The most abundant metals measured were Fe (5.17 μg Fe/6.1 μg total metal deposition = 84.75%) and Mn (0.87 μg Mn/6.1 μg total metal deposition = 14.26%), which equates to the elemental analysis of the GMAW-MS fume shown in Table 1.
Table 2.
Lung metal deposition in A/J mice after air or GMAW-MS fume whole-body inhalation for 4 hours at a concentration of 36.4 mg/m3. Freeze-dried whole lung tissue was analyzed for aluminum (Al), chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), titanium (Ti), and zinc (Zn) by Inductively Coupled Plasma-Atomic Emission Spectroscopy. Samples were prepared according to the NIOSH method 7300 for bulk tissue samples. Levels of Al, Cr, Ni, Ti, and Zn were not significantly increased in exposed animals or not detected. Note: Values are means ± standard error (n = 10 air; n = 10 GMAW-MS). Abbreviations: GMAW-MS – gas metal arc welding-mild steel.
| Exposure | Fe (μg/lung) | Mn (μg/lung) | Cu (μg/lung) |
|---|---|---|---|
| Air | 9.45 ± 0.29 | 0.01 ± 0.00 | 0.23 ± 0.01 |
| GMAW-MS | 14.62 ± 0.45 | 0.88 ± 0.05 | 0.29 ± 0.01 |
3.2.1. Human relevance deposition calculation
The analysis of the metals showed a cumulative increase of 6.1 μg of total GMAW-MS fume deposited in the lung from a single 4 hour exposure (Table 2). The alveolar deposition in the mice was equated to the human by the equations below using the previous threshold limit value-time weighted average (TLV-TWA) of 5 mg/m3 for total welding fume. Previously, it was estimated that 70% of the total dose reached the alveolar space (6.1 μg/d x 0.70 = 4.27 μg/d) (Erdely et al., 2011; Raabe et al., 1988). The mice were exposed for 32 days (8 weeks at 4 days/week) for an approximate total alveolar deposition of 136.64 μg.
3.2.2. Estimated human daily deposition using previous welding fume TLV-TWA of 5 mg/m3
Fume concentration x min volume x exposure duration x deposition efficiency = deposited human dose
| 5 mg/m3 x (20 l/min)(10−3 m3/l) x (8 hours/day)(60 min/hour) x 0.16 = 7.7 mg deposited per 8 hour day in humans |
3.2.3. Estimated human equivalent deposition from quantified deposition in mouse using alveolar surface area (SA) (Stone et al., 1992)
278.75 mg/7.7 mg/day = approximately 36 working days for a human working at 5 mg/m3 for 8 hours/day. While it is understood that welding is usually not done for 8 hours/day, and the exposure levels are likely not to consistently reach 5 mg/m3 as a TWA, the deposition in this study model was representative of cumulative exposure in a human.
3.3. Morbidity and mortality
Initial body weights at week 0 (means ± standard error) were 19.24 ± 0.30, 19.10 ± 0.28, 19.41 ± 0.27, and 19.18 ± 0.28 g for the CO/air, CO/GMAW-MS, MCA/air, and MCA/GMAW-MS groups, respectively. Body weights were not significantly changed due to exposure and increased steadily from week 0 to 30 with average net weight gains of 10.82, 10.28, 10.25, and 10.91 g for the CO/air, CO/ GMAW-MS, MCA/air, and MCA/GMAW-MS groups, respectively. Morbidity and mortality throughout the study was low (∼5%) and no abnormalities, such as other tumor types besides lung, were found at the terminal sacrifice at 30 weeks. In total, 6 mice died during the course of the study and were not included in the final analysis of the data. Necropsy determined that all 6 mice died from morbidities that included groin-associated skin lesions or a cause of death otherwise undetermined but not associated with the experimental protocol.
3.4. Gross tumor multiplicity and incidence
The total tumor number per mouse lung for each exposure group (horizontal lines indicate gross-observed tumor multiplicity) is shown in Fig. 3. GMAW-MS fume significantly promoted lung tumors in mice at 30 weeks after initiation with MCA. Lung tumor multiplicity was 8.34 ± 0.59 and 21.86 ± 1.50 for MCA/air and MCA/GMAW-MS, respectively (p < 0.0001). There was no effect of welding fume alone on tumor multiplicity (CO/air, 0.28 ± 0.11; CO/GMAW-MS, 0.18 ± 0.07; p = 0.44). Average lung tumor incidence (% of tumor- bearing mice) was 21% in CO/air and 17% in CO/GMAW-MS-exposed animals. Reports in the literature indicate the background lung tumor incidence in A/J mice between 43 and 53 weeks of age to be 31–40% (Groch et al., 1997; Curtin et al., 2004). The mice in this study were 35 or 36 weeks of age upon sacrifice, indicating the observed tumor incidence is consistent with reported findings. As expected, incidence was 100% in all MCA-initiated groups (n = 29 for MCA/air and n = 28 MCA/GMAW-MS groups), which confirmed the successful administration as well as its carcinogenic effectiveness in A/J mice. Total and average tumor number per treatment group across each of the individual lung lobes is reported in Table 3. MCA/GMAW-MS fume-exposed mice had significantly greater lung tumor multiplicity in every lung region compared to MCA/air (p < 0.05).
Fig. 3. Tumor number per mouse lung and tumor multiplicity upon gross examination in A/J mice following initiation-promotion study at 30 week sacrifice.
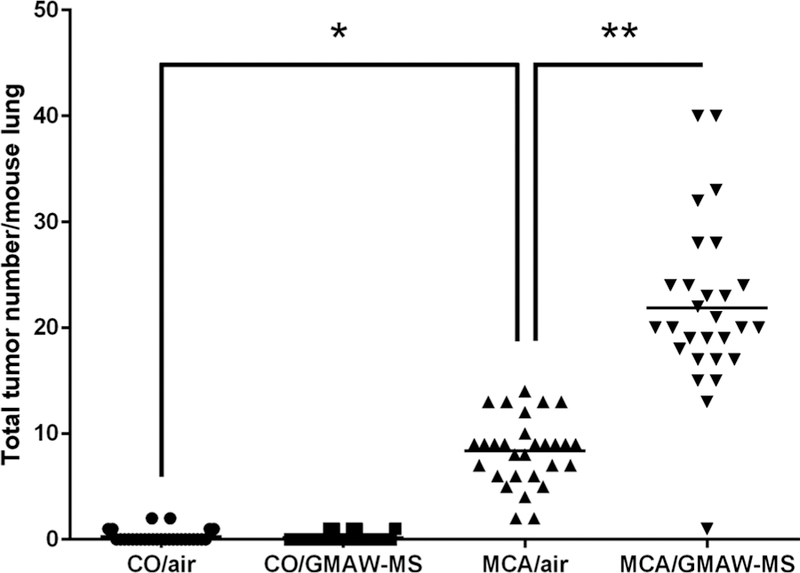
MCA initiation followed by GMAW-MS fume inhalation significantly increased lung tumor multiplicity compared to MCA/air (21.86 ± 1.50 vs 8.34 ± 0.59, respectively). As expected, mice not initiated with MCA had low tumor numbers. Horizontal bars represent mean tumor numbers per group. Circles = CO/air, squares = CO/GMAW-MS, upward triangles = MCA/air, downward triangles = MCA/GMAW-MS. *p < 0.0001 – compared to CO/air; **p < 0.0001 – compared to MCA/air.
Table 3.
Total and means ± standard error () tumor number in A/J mice across each of the individual lung lobes following GMAW-MS fume or air whole-body inhalation exposure 30 weeks post-initiation with MCA or corn oil.
| n | Left | Apical | Cardiac | Diaphragmatic | Azygos | |
|---|---|---|---|---|---|---|
| CO/air | 29 | 4 (0.14 ± 0.07) | 2 (0.07 ± 0.05) | 0 | 1 (0.03 ± 0.03) | 1 (0.03 ± 0.03) |
| CO/GMA-MS | 28 | 3 (0.12 ± 0.06) | 0 | 0 | 1 (0.04 ± 0.04) | 1 (0.04 ± 0.04) |
| MCA/air | 29 | 81 (2.79 ± 0.32)** | 28 (0.97 ± 0.16)** | 37 (1.28 ± 0.24)** | 67 (2.31 ± 0.29)** | 29 (1.00 ± 0.16)** |
| MCA/GMA-MS | 28 | 226 (8.07 ± 0.74)* | 77 (2.75 ± 0.31)* | 77 (2.75 ± 0.36)* | 187 (6.68 ± 0.48)* | 45 (1.61 ± 0.28)^ |
Abbreviations: CO- corn oil; GMAW-MS- gas metal arc welding – mild steel; MCA-3-methylchloanthrene.
p < 0.0001 - compared to MCA/air
p < 0.0001 - compared to CO/air
p < 0.04 - compared to MCA/air.
Gross lung morphology from a GMAW-MS fume-exposed mouse initiated with MCA is shown in Fig. 4. Welding fume deposition was visible in all exposed mouse lungs and appeared reddish to black-brown in color. Tumors appeared white in color and opaque on initial gross exam and became more well-defined after fixation which aided enumeration. At 30 weeks, tumors were between ∼0.5 mm and ∼4 mm in diameter, with most tumors ∼1 mm. Mean tumor sizes were 1.14 ± 0.12, 0.98 ± 0.20, 1.14 ± 0.02, and 1.19 ± 0.02 mm for CO/air, CO/GMAW-MS, MCA/air, and MCA/GMAW-MS, respectively.
Fig. 4. Gross images of a representative A/J mouse lung promoted by GMAW-MS fume at 30 weeks post-initiation with MCA.
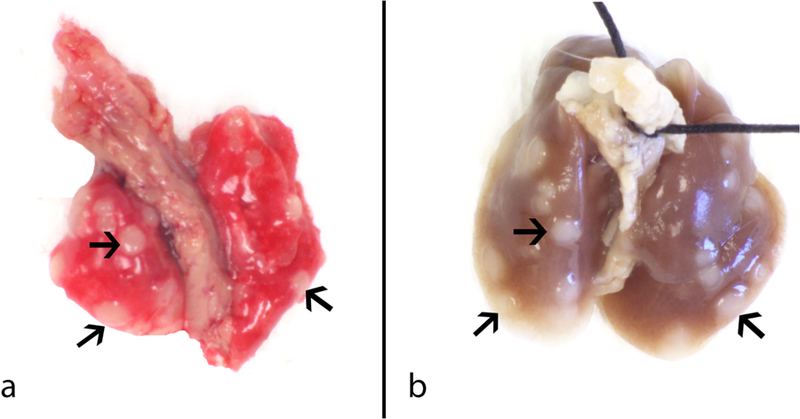
Panel A – Lung tumor morphology before fixation. Panel B – Lung tumors 24 h after fixation. Tumors (arrows) were on average ∼1 mm in diameter and opaque in color.
3.5. Histopathological evaluation of lung lesions, inflammation, and welding fume presence
Severity scores for abnormal morphological findings and numbers of lung lesions observed in lung sections from A/J mice are shown in Table 4. Histopathology analysis confirmed the gross findings, with significantly greater lung tumor multiplicity in the MCA/GMAW-MS animals (9.86 ± 0.88) compared to MCA/air (3.34 ± 0.34), CO/ GMAW-MS (0.14 ± 0.07), or CO/air animals (0.27 ± 0.12). In addition, two bronchioloalveolar adenocarcinomas were present in the MCA/GMAW-MS-exposed mice. None were observed in other treatment groups. Bronchiolo-alveolar adenomas and adenocarcinomas can arise from foci of alveolar hyperplasia (Renne et al., 2009). Some of the adenomas in this study were solid discrete nodular masses, typical of adenoma, but the majority of the lesions diagnosed as adenomas arose within hyperplasias. Hyperplasias generally had irregular borders and consisted of alveoli lined by plump, round to ovoid to cuboidal epithelial cells that formed a single layer and, occasionally, small foci of hypercellularity, but still maintained the normal alveolar architecture (Fig. 5A and 5B). Lesions were distinguished by focal nodular areas characterized by abnormal growth structure characteristic of adenoma, such as solid hypercellular areas and hypercellular papillary structures sometimes containing slightly atypical cells, that were clearly different from the adjacent areas of hyperplasia, resulting in disruption of the normal alveolar architecture (Fig. 5C and 5D). This represented transition from hyperplasia to neoplasia and these lesions were diagnosed simply as bronchioloalveolar adenoma.
Table 4.
Lung histopathological findings including number of microscopically observed lung lesions at 30 weeks post-initiation with MCA or CO in A/J mice exposed to GMAW-MS fume or filtered air by whole-body inhalation.
| n | Foreign Material^ | Hyperplasia Severity^ | Hyperplasia# | Adenoma# | Adenocarcinoma# | Total Lesions# | |
|---|---|---|---|---|---|---|---|
| CO/air | 15 | – | 0.1 ± 0.05 | 0.2 ± 0.11 (3) | 0.07 ± 0.07 (1) | – | 0.27 ± 0.12 (4) |
| CO/GMAW-MS | 28 | 0.91 ± 0.05** | 0.02 ± 0.02 | 0.07 ± 0.05 (2) | 0.07 ± 0.05 (2) | – | 0.14 ± 0.07 (4) |
| MCA/air | 29 | 0.02 ± 0.02 | 1.79 ± 0.18** | 2.93 ± 0.34 (85)** | 0.41 ± 0.13 (12)ǂ | – | 3.34 ± 0.34 (97)** |
| MCA/GMAW-MS | 28 | 0.96 ± 0.02* | 2.55 ± 0.16* | 7.11 ± 0.64 (199)* | 2.67 ± 0.36 (75)* | 0.07 ± 0.05 (2) | 9.86 ± 0.86 (276)* |
indicates no findings.
Severity scores are the averages of the left and right lung lobes and are presented as means ± standard error. Foreign material refers to presence of presumptive GMAW-MS fume (brown-black pigment) in the lungs. Severity was scored as 1 = minimal, 2 = mild, 3 = moderate, 4 = marked.
Hyperplasias, adenomas, and adenocarcinomas are presented as mean ± standard error with total lesion number in parenthesis.
Abbreviations: GMAW-MS – gas metal arc welding – mild steel; MCA – 3 – methylcholanthrene; CO – corn oil.
p < 0.0001 compared to MCA/air
p < 0.0001 compared to CO/air
p < 0.02 compared to CO/air.
Fig. 5. Photomicrographs of lung tissue from MCA/GMAW-MS fume-exposed mice.
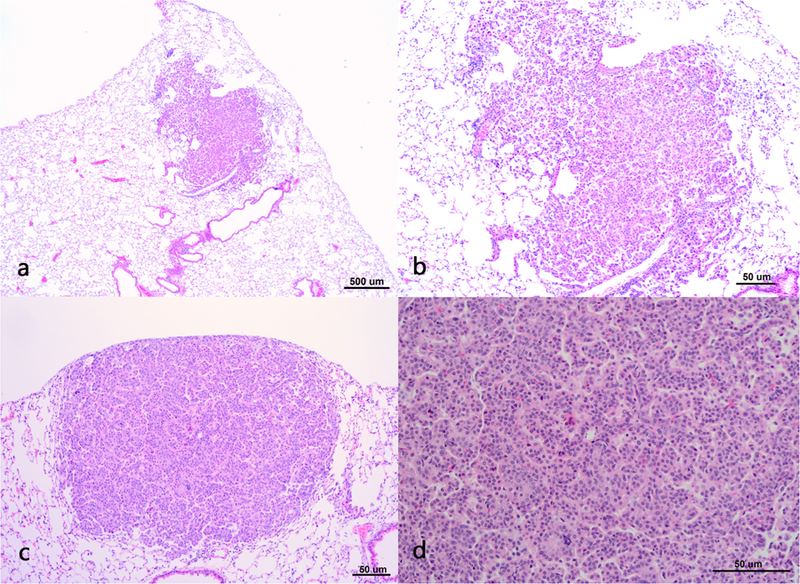
Panel A- moderate bronchioloalveolar hyperplasia, 4x magnification. Panel B – moderate bronchioloalveolar hyperplasia,10x magnification. Panel C – bronchioloalveolar adenoma as a solid discrete mass, 10x magnification. Panel D - bronchioloalveolar adenoma, 20x magnification.
Foreign material, (i.e., GMAW-MS fume), was observed in the right and left lobes of nearly all MCA/GMAW-MS and CO/GMAW-MS fume-exposed lungs. The foreign material ranged in appearance from multiple, scattered small clusters of minute discrete focal aggregates of histiocytes to a few widely scattered individual histiocytes containing black intracytoplasmic granules. Foreign material was graded based upon the amount of accumulated material present and was considered minimal in all cases. Unremarkable inflammation was observed in any of the treatment groups. No significant monocytic or lymphoid infiltrate was observed in any mouse lungs, indicating a lack of inflammation from GMAW-MS fume inhalation exposure.
3.6. BAL findings at different time points post-exposure to GMAW-MS fume
At 1 day post-exposure, cytotoxicity was not significantly increased in GMAW-MS fume-exposed compared to air-exposed mice (111.10 ± 7.08 and 101.90 ± 4.65, respectively). The same result was found at 7, 28, and 84 days post-exposure (Table 5). In both the GMAW-MS fume- and air-exposed groups, cell populations were > 99% macrophages. Similarly, slides from air-exposed animals typically contained > 99% macrophages. A significant increase in macrophage cell number was seen in GMAW-MS fume-exposed mice at 28 and 84 days post-exposure (p < 0.05). No significant neutrophils, eosinophils, or lymphocytes were observed at any time point post-exposure.
Table 5.
Bronchoalveolar lavage parameters after whole-body inhalation exposure to air or GMAW-MS fume at an average concentration 36.28 mg/m3 for 4 h/day for 10 days. Differentials based on cell count of > 300 cells. Lymphocytes, neutrophils, and eosinophils were absent in the lavage and excluded from the table. Note: Values are means ± standard error. Abbreviations: GMAW-MS - gas metal arc welding - mild steel; LDH – lactate dehydrogenase; BAL- bronchoalveolar lavage.
| Exposure | Time Point (days) | n | LDH (U/L) | Macrophages (BAL cell #/ml) |
|---|---|---|---|---|
| Air | 1 | 8 | 101.90 ± 4.65 | 414286 ± 23234 |
| GMAW-MS | 8 | 111.10 ± 7.08 | 442500 ± 40820 | |
| Air | 7 | 8 | 91.80 ± 4.06 | 452500 ± 28800 |
| GMAW-MS | 8 | 100.50 ± 2.86 | 411021 ± 33726 | |
| Air | 28 | 8 | 84.30 ± 4.42 | 282500 ± 54052 |
| GMAW-MS | 8 | 85.10 ± 6.00 | 491250 ± 67,445* | |
| Air | 84 | 8 | 103.70 ± 6.57 | 327143 ± 47193 |
| GMAW-MS | 8 | 84.50 ± 2.41 | 520000 ± 56,901* |
p < 0.05.
4. Discussion
It was found that GMAW-MS fume is a lung tumor promoter in A/J mice, despite a lack of significant chronic lung inflammation or the presence of known carcinogenic metals in the fume. Gross and histopathological examination revealed significantly increased lung tumor multiplicity in MCA/GMAW-MS fume-exposed mice compared to MCA/ air. These results support epidemiological findings that MS welders are at increased risk of lung cancer despite absence of known carcinogens in the MS fume (Hansen and Lauritsen, 1996; Lauritsen and Hansen, 1996). The promotion effect was achieved at a lower fume deposition than previous GMAW-SS oropharyngeal aspiration or inhalation studies (Falcone et al., 2017; Zeidler-Erdely et al., 2013). Whole lung metal analysis revealed 6.1 μg of GMAW-MS fume was deposited after a single 4 h exposure, which equates to an approximate total deposition of μg in the alveolar region of the lung throughout the timecourse. A previous GMAW-SS fume inhalation study by Falcone et al. found a daily lung metal deposition of ∼10.1 μg, an approximate total deposition of 254.4 μg in the alveolar region (Falcone et al., 2017). The GMAW-MS fume deposition was approximately 46% lower in comparison, yet the tumor multiplicity was similar (GMAW-MS, 21.86 ± 1.50; GMAW-SS, 16.11 ± 1.18).
The GMAW-MS fume contains both Mn and Fe, unlike GMAW-SS fume that contains known carcinogenic metals such as hexavalent chromium. There is no clear evidence that Mn may be carcinogenic nor does it have a current cancer classification. Studies suggest Mn as having primarily neurotoxic effects. These include Manganism and a Parkinson disease-like disorder in welders (Antonini, 2014; Laohaudomchok et al., 2011; Roels et al., 2012) and impairment in learning and memory in rats (Liang et al., 2015; Shukakidze et al., 2003). In contrast, it is known that excess Fe accumulation in the body increases cancer risk. Evidence from diseases such as hemochromatosis, mylodysplastic syndromes, and endometriosis demonstrate that Fe overloading leads to the development of cancer (Manz et al., 2016; Steegmann-Olmedillas, 2011; Zhang and Zhang, 2015; Jiang et al., 2016). Fe may also play an indirect role in the pathogenesis of asbestos-induced mesothelioma (Jiang et al., 2016). Although Fe is a crucial micronutrient for the body, too much Fe may increase cancer risk through the production of reactive oxygen species as one potential mechanism. The Fe in welding fume is present as iron oxides, including Fe2O3. Once inhaled, this Fe3+ could be reduced and then oxidized by the Fenton reaction to create hydroxyl radical possibly leading to DNA damage. Hydroxyl radical has also been shown to accelerate migratory and invasive capabilities of lung cancer cells and modulate signals that regulate cell transformation, increase apoptosis, and alter gene expression, although it is unclear if this is occurring in this study given the lack of overt inflammation (Steegmann-Olmedillas, 2011; Luanpitpong et al., 2010). Altered Fe homeostasis has been suggested to be associated adverse pulmonary outcomes including cancer (Manz et al., 2016; Ghio and Hilborn, 2017). Epidemiological evidence from numerous worker studies involving industrial exposures to iron oxides, such as iron ore mining, iron and steel founding, and welding, reveal increased cancer risks in these populations (Boyd et al., 1970; Wild et al., 2009). However, the results are conflicting, with some reports suggesting no increased cancer risk (Bourgkard et al., 2009). Iron oxide exposures are difficult to study in isolation, though, as many of these occupations also involve exposures to other cancer-causing materials such as radon, cigarette smoke, or carcinogenic metals. Furthermore, in vivo iron oxide inhalation studies are scarce (Kornberg et al., 2017). Because of limited evidence from “pure” human iron oxide exposures, iron oxide is currently not classified as carcinogenic to humans according to the IARC (Wild et al., 2009).
No signficant lung inflammation or cytotoxicity was found in this study after a short-term GMAW-MS fume exposure. Histopathological analysis showed no evidence of chronic lung inflammation. These findings agree with earlier occupational and rat mild steel welding fume studies (Antonini et al., 2009; Antonini, 2003). Substantial evidence indicates that tumor promotion and inflammation are often connected, and inflammation is considered an enabling hallmark of carcinogenesis (Hanahan and Weinberg, 2011; Fujiki et al., 2013, 2000). Interestingly, GMAW-MS fume exhibited a similar lung tumor promotion potential as GMAW-SS fume which is known to cause persistent pulmonary inflammation, indicating additional or alternative mechanisms (Zeidler-Erdely et al., 2008). Many carcinogens are now thought to cause both genetic and/or epigenetic changes that may lead to cancer. It is possible there may be an epigenetic effect occurring with welding fume exposures (Herceg et al., 2013). A number of metals such as nickel, cadmium, and arsenic, as well as exposure to substances like alcohol and cigarette smoke, are known to cause epigenetic changes such as DNA methylation and histone modifications which can lead to cancer (Herceg et al., 2013; Chappell et al., 2016). Fan T. et al. observed an epigenetic response of transposable elements after short term exposure to high-dose welding particulate matter (Fan et al., 2014). In particular, they found that welding particulate matter was associated with increased blood methylation levels of LINE-1. Additionally, stainless steel welding fume exposure has been shown to cause telomere length changes in circulating peripheral blood monocytes of rats (Shoeb et al., 2017). Epigenetic changes are now considered an important contributor to carcinogenesis which will be important to investigate with welding fumes in the future.
The immunosuppressive effects of welding fume exposure may be a contributing mechanism for the resulting lung carcinogenesis. Worker studies have indicated that welders are at increased risk of lobar pneumonia from Streptococcus pneumoniae infection as well as other lung infections (Coggon et al., 1994; Coggon and Palmer, 2016; Suri et al., 2016; Zeidler-Erdely et al., 2012). A cross-sectional study of shipyard workers in the Middle East found that those exposed to welding fumes had a higher prevalence of respiratory symptoms and were more likely to report to healthcare professionals concerning respiratory infections (Marongiu et al., 2016). Epidemiological studies have found that welders are at increased risk of developing and dying from pneumococcal and lobar pneumonia, suggesting a need for pneumococcal vaccination among welders (Coggon et al., 2015). In vivo studies have corrobated the immunosuppressive effects of welding fumes, including a rat inhalation exposure to the GMAW-MS fume used in this study (Antonini et al., 2009; Zeidler-Erdely et al., 2012). According to prevailing hypotheses, the reasons for increased risk of infection in welders include the ability of Fe to act as a micronutrient for bacteria, inhibition of the immune system, and enhanced binding of Streptococcus pneumoniae to lung epithelial cells (Coggon and Palmer, 2016). Recent research most strongly supports enhanced bacterial binding to lung epithelial cells as the primary mechanism for the increased Streptococcal infection risk (Coggon et al., 1994; Suri et al., 2016; Wong et al., 2010). If GMAW-MS fume suppressed mouse lung defenses in this study, it is possible this represents a potential mechanism by which tumor promotion is occurring.
In conclusion, this study is the first to demonstrate that GMAW-MS fume, despite containing no metals currently classified as carcinogens, promotes lung tumors in A/J mice. Future studies in our laboratory will investigate the toxicity and tumorigenic potential of the individual metal components of GMAW-SS and GMAW-MS fumes. Results of these studies may help to identify the metal components that are most toxic and tumorigenic. Ultimately, a more complete understanding of the carcinogenic potential of the welding fume components can lead to a safer work environment for welders.
Footnotes
Conflicts of interest
The authors declare they have no conflicts of interest.
Human and animal rights
All animal studies have been approved by the NIOSH Animal Care and Use Committee (ACUC) and all applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All animal experiments comply with the ARRIVE guidelines.This article does not contain any studies with human participants performed by any of the authors.
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
References
- Akatsuka S, Toyokuni S, 2016. Iron function and carcinogenesis. Nihon Rinsho 74, 1168–1175. [PubMed] [Google Scholar]
- Andrews NC, 2000. Iron homeostasis: insights from genetics and animal models. Nat. Rev. Genet 1, 208–217. [DOI] [PubMed] [Google Scholar]
- Antonini JM, 2003. Health effects of welding. Crit. Rev. Toxicol 33, 61–103. [DOI] [PubMed] [Google Scholar]
- Antonini JM, 2014. Health effects associated with welding. In: In: Bassim N (Ed.), Comprehensive Materials Processing, vol. 8 Elsevier Ltd., pp. 49–70. [Google Scholar]
- Antonini JM, Afshari AA, Stone S, Chen B, Schwegler-Berry D, Fletcher WG, Goldsmith WT, Vandestouwe KH, McKinney W, Castranova V, Frazer DG, 2006. Design, construction, and characterization of a novel robotic welding fume generator and inhalation exposure system for laboratory animals. J. Occup. Environ. Hyg 3, 194–203 quiz D145. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Stone S, Roberts JR, Chen B, Schwegler-Berry D, Afshari AA, Frazer DG, 2007. Effect of short-term stainless steel welding fume inhalation exposure on lung inflammation, injury, and defense responses in rats. Toxicol. Appl. Pharmacol 223, 234–245. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Stone S, Chen BT, Schwegler-Berry D, Frazer DG, 2009. Short-term inhalation exposure to mild steel welding fume had no effect on lung inflammation and injury but did alter defense responses to bacteria in rats. Inhal. Toxicol 21, 182–192. [DOI] [PubMed] [Google Scholar]
- Bourgkard E, Wild P, Courcot B, Diss M, Ettlinger J, Goutet P, Hemon D, Marquis N, Mur JM, Rigal C, et al. , 2009. Lung cancer mortality and iron oxide exposure in a French steel-producing factory. Occup. Environ. Med 66, 175–181. [DOI] [PubMed] [Google Scholar]
- Boyd JT, Doll R, Faulds JS, 1970. Leiper J: Cancer of the lung in iron ore (haematite) miners. Br. J. Ind. Med 27, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JA, 1940. Effects of Precipitated Silica and of Iron Oxide on the Incidence of Primary Lung Tumours in Mice. Br. Med. J 2, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell G, Pogribny IP, Guyton KZ, Rusyn I, 2016. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: a systematic literature review. Mutat. Res. Rev. Mutat. Res 768, 27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggon D, Palmer KT, 2016. Are welders more at risk of respiratory infections? Thorax 71, 581–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggon D, Inskip H, Winter P, Pannett B, 1994. Lobar pneumonia: an occupational disease in welders. Lancet 344, 41–43. [DOI] [PubMed] [Google Scholar]
- Coggon D, Harris EC, Cox V, Palmer KT, 2015. Pneumococcal vaccination for welders. Thorax 70, 198–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin GM, Higuchi MA, Ayres PH, Swauger JE, Mosberg AT, 2004. Lung tumorigenicity in A/J and rasH2 transgenic mice following mainstream tobacco smoke inhalation. Toxicol. Sci 81, 26–34. [DOI] [PubMed] [Google Scholar]
- Erdely A, Hulderman T, Salmen-Muniz R, Liston A, Zeidler-Erdely PC, Chen BT, Stone S, Frazer DG, Antonini JM, Simeonova PP, 2011. Inhalation exposure of gas-metal arc stainless steel welding fume increased atherosclerotic lesions in apolipoprotein E knockout mice. Toxicol. Lett 204, 12–16. [DOI] [PubMed] [Google Scholar]
- Falcone LM, Erdely A, Meighan TG, Battelli LA, Salmen R, McKinney W, Stone S, Cumpston A, Cumpston J, Andrews RN, et al. , 2017. Inhalation of gas metal arc-stainless steel welding fume promotes lung tumorigenesis in A/J mice. Arch. Toxicol 91, 2953–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Fang SC, Cavallari JM, Barnett IJ, Wang Z, Su L, Byun HM, Lin X, Baccarelli AA, Christiani DC, 2014. Heart rate variability and DNA methylation levels are altered after short-term metal fume exposure among occupational welders: a repeated-measures panel study. BMC Public Health 14, 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Okabe S, Sueoka E, Suga K, Imai K, Nakachi K, 2000. A new concept of tumor promotion by tumor necrosis factor-alpha, and cancer preventive agents (−)-epigallocatechin gallate and green tea–a review. Cancer Detect. Prev 24, 91–99. [PubMed] [Google Scholar]
- Fujiki H, Sueoka E, Suganuma M, 2013. Tumor promoters: from chemicals to inflammatory proteins. J. Cancer Res. Clin. Oncol 139, 1603–1614. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Hilborn ED, 2017. Indices of iron homeostasis correlate with airway obstruction in an NHANES III cohort. Int. J. Chron. Obstruct. Pulmon. Dis 12, 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groch KM, Khan MA, Brooks AL, Saffer JD, 1997. Lung cancer response following inhaled radon in the A/J and C57BL/6J mouse. Int. J. Radiat. Biol 71, 301–308. [DOI] [PubMed] [Google Scholar]
- Guha N, Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Vilahur N, Muller K, Straif K, 2017. Carcinogenicity of welding, molybdenum trioxide, and indium tin oxide. Lancet Oncol 18, 581–582. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hansen KS, Lauritsen JM, 1996. Skytthe A: Cancer incidence among mild steel and stainless steel welders and other metal workers. Am. J. Ind. Med 30, 373–382. [DOI] [PubMed] [Google Scholar]
- Herceg Z, Lambert MP, van Veldhoven K, Demetriou C, Vineis P, Smith MT, Straif K, 2013. Wild CP: towards incorporating epigenetic mechanisms into carcinogen identification and evaluation. Carcinogenesis 34, 1955–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Chew SH, Nakamura K, Ohara Y, Akatsuka S, Toyokuni S, 2016. Dual preventive benefits of iron elimination by desferal in asbestos-induced mesothelial carcinogenesis. Cancer Sci 107, 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg TG, Stueckle TA, Antonini JA, Rojanasakul Y, Castranova V, Yang Y, 2017. Wang l: potential toxicity and underlying mechanisms associated with pulmonary exposure to Iron oxide nanoparticles: conflicting literature and unclear risk. Nanomaterials (Basel) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Shrairman R, Landau A, Christiani DC, Weisskopf MG, 2011. Neuropsychological effects of low-level manganese exposure in welders. Neurotoxicology 32, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen JM, Hansen KS, 1996. Lung cancer mortality in stainless steel and mild steel welders: a nested case-referent study. Am. J. Ind. Med 30, 383–391. [DOI] [PubMed] [Google Scholar]
- Liang G, Qin H, Zhang L, Ma S, Huang X, Lv Y, Qing L, Li Q, Xiong Y, Huang Y, et al. , 2015. Effects of chronic manganese exposure on the learning and memory of rats by observing the changes in the hippocampal cAMP signaling pathway. Food Chem. Toxicol 83, 261–267. [DOI] [PubMed] [Google Scholar]
- Luanpitpong S, Talbott SJ, Rojanasakul Y, Nimmannit U, Pongrakhananon V, Wang L, Chanvorachote P, 2010. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J. Biol. Chem 285, 38832–38840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz DH, Blanchette NL, Paul BT, Torti FM, Torti SV, 2016. Iron and cancer: recent insights. Ann. N. Y. Acad. Sci 1368, 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marongiu A, Hasan O, Ali A, Bakhsh S, George B, Irfan N, Minelli C, Canova C, Schofield S, De Matteis S, Cullinan P, 2016. Are welders more at risk of respiratory infections? Findings from a cross-sectional survey and analysis of medical records in shipyard workers: the WELSHIP project. Thorax 71, 601–606. [DOI] [PubMed] [Google Scholar]
- Matrat M, Guida F, Mattei F, Cenee S, Cyr D, Fevotte J, Sanchez M, Menvielle G, Radoi L, Schmaus A, et al. , 2016. Welding, a risk factor of lung cancer: the ICARE study. Occup. Environ. Med 73, 254–261. [DOI] [PubMed] [Google Scholar]
- Pease C, Rucker T, Birk T, 2016. Review of the evidence from epidemiology, toxicology, and lung bioavailability on the carcinogenicity of inhaled iron oxide particulates. Chem. Res. Toxicol 29, 237–254. [DOI] [PubMed] [Google Scholar]
- Raabe OG, Al-Bayati MA, Teague SV, Rasolt A, 1988. Regional deposition of inhaled monodisperse coarse and fine aerosol particles in small laboratory animals. Ann. Occup. Hyg 32, 53–63. [Google Scholar]
- Rehm S, Ward JM, 1989. Quantitative analysis of alveolar type II cell tumors in mice by whole lung serial and step sections. Toxicol. Pathol 17, 737–742. [DOI] [PubMed] [Google Scholar]
- Renne R, Brix A, Harkema J, Herbert R, Kittel B, Lewis D, March T, Nagano K, Pino M, Rittinghausen S, et al. , 2009. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol. Pathol 37, 5s–73s. [DOI] [PubMed] [Google Scholar]
- Roels HA, Bowler RM, Kim Y, Claus Henn B, Mergler D, Hoet P, Gocheva VV, Bellinger DC, Wright RO, Harris MG, et al. , 2012. Manganese exposure and cognitive deficits: a growing concern for manganese neurotoxicity. Neurotoxicology 33, 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Kodali VK, Farris BY, Bishop LM, Meighan TG, Salmen R, Eye T, Friend S, Schwegler-Berry D, Roberts JR, et al. , 2017. Oxidative stress, DNA methylation, and telomere length changes in peripheral blood mononuclear cells after pulmonary exposure to metal-rich welding nanoparticles. NanoImpact 5, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukakidze A, Lazriev I, Mitagvariya N, 2003. Behavioral impairments in acute and chronic manganese poisoning in white rats. Neurosci. Behav. Physiol 33, 263–267. [DOI] [PubMed] [Google Scholar]
- Sorensen AR, Thulstrup AM, Hansen J, Ramlau-Hansen CH, Meersohn A, Skytthe A, Bonde JP, 2007. Risk of lung cancer according to mild steel and stainless steel welding. Scand. J. Work Environ. Health 33, 379–386. [DOI] [PubMed] [Google Scholar]
- Steegmann-Olmedillas JL, 2011. The role of iron in tumour cell proliferation. Clin. Transl. Oncol 13, 71–76. [DOI] [PubMed] [Google Scholar]
- Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD, 1992. Allometric relationships of cell numbers and size in the mammalian lung. Am. J. Respir. Cell Mol. Biol 6, 235–243. [DOI] [PubMed] [Google Scholar]
- Suri R, Periselneris J, Lanone S, Zeidler-Erdely PC, Melton G, Palmer KT, Andujar P, Antonini JM, Cohignac V, Erdely A, et al. , 2016. Exposure to welding fumes and lower airway infection with Streptococcus pneumoniae. J. Allergy Clin. Immunol 137, 527–534 e527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Roberts JR, Leonard SS, Shi X, Antonini JM, 2003. Effects of welding fumes of differing composition and solubility on free radical production and acute lung injury and inflammation in rats. Toxicol. Sci 75, 181–191. [DOI] [PubMed] [Google Scholar]
- The Procedure Handbook of Arc Welding, 14 edn. The James F. Lincoln Arc Welding Foundation. [Google Scholar]
- Wild P, Bourgkard E, Paris C, 2009. Lung cancer and exposure to metals: the epidemiological evidence. Methods Mol. Biol 472, 139–167. [DOI] [PubMed] [Google Scholar]
- Wong A, Marrie TJ, Garg S, Kellner JD, Tyrrell GJ, 2010. Welders are at increased risk for invasive pneumococcal disease. Int. J. Infect. Dis 14, e796–799. [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Kashon ML, Battelli LA, Young SH, Erdely A, Roberts JR, Reynolds SH, Antonini JM, 2008. Pulmonary inflammation and tumor induction in lung tumor susceptible A/J and resistant C57BL/6J mice exposed to welding fume. Part. Fibre Toxicol 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Battelli LA, Stone S, Chen BT, Frazer DG, Young SH, Erdely A, Kashon ML, Andrews R, Antonini JM, 2011. Short-term inhalation of stainless steel welding fume causes sustained lung toxicity but no tumorigenesis in lung tumor susceptible A/J mice. Inhal. Toxicol 23, 112–120. [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Erdely A, Antonini JM, 2012. Immunotoxicology of arc welding fume: worker and experimental animal studies. J. Immunotoxicol 9, 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Meighan TG, Erdely A, Battelli LA, Kashon ML, Keane M, Antonini JM, 2013. Lung tumor promotion by chromium-containing welding particulate matter in a mouse model. Part. Fibre Toxicol 10, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang F, 2015. Iron homeostasis and tumorigenesis: molecular mechanisms and therapeutic opportunities. Protein Cell 6, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]


