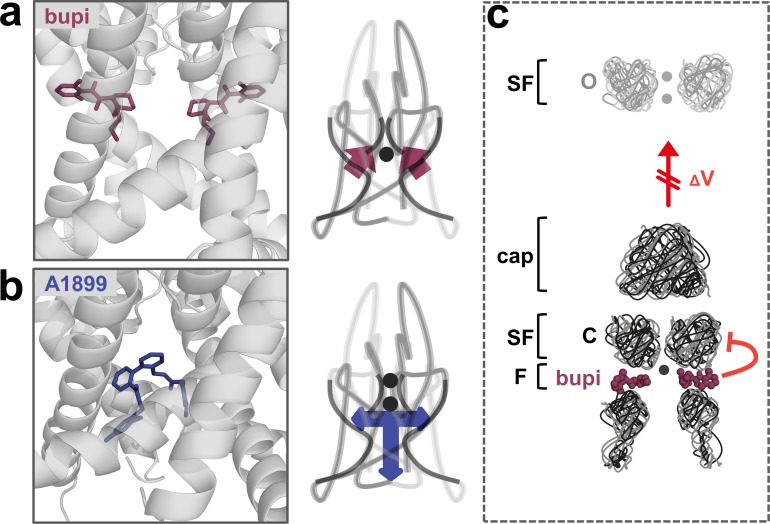
Figure 6. A novel allosteric inhibition mechanism interferes with the voltage-dependent activation of TASK channels.
(a) Illustration of the bupivacaine binding mode in the side fenestrations (left), preventing K+-flux gating, which requires a K+ filled selectivity filter (right cartoon). The closed state or collapsed selectivity filter is indicated by one potassium ion (black dot). (b) A1899 located in the central cavity (left) is binding in an 'anchor'-shaped like structure which occludes the pore and thus prevents K+ permeation (right cartoon). Here the K+ occupancy of the selectivity filter is not disturbed (illustrated by two black dots in the selectivity filter). (c) Schematic illustration of the proposed gating model of TASK-1. The upper panel illustrates the selectivity filter in the open state and the lower panel the TASK channel. 'C' indicates the closed state conformation of the selectivity filter (one potassium ion located before the collapsed filter) and 'O' indicates the open state with a K+ filled selectivity filter (here two potassium ions illustrated). SF indicates the selectivity filter and F the side fenestrations. The voltage-dependent (ΔV) opening of the selectivity filter gate (K+-flux gating) preferentially results in the typical outwardly rectifying K+ currents. The presence of bupivacaine in the side fenestrations prevents the voltage-dependent K+-flux gating, resulting in reduced outward currents at depolarized potentials and thus a voltage-dependent inhibition.